DOI:
10.1039/C6RA09619K
(Paper)
RSC Adv., 2016,
6, 63519-63524
Dispersion polymerization of acrylamide with living character and controlled morphologies initiated and mediated by cobalt porphyrin
Received
14th April 2016
, Accepted 22nd June 2016
First published on 23rd June 2016
Abstract
Dispersion polymerization of acrylamide (AM) catalyzed by cobalt porphyrin [cobalt tetramethoxyphenylporphyrin, (TMOP)Co(II)] with the feature of living radical polymerization (LRP) in alcohol/water or dimethylformamide (DMF)/water is described. Well-defined polyacrylamide (PAM) uniform spherical particles with predetermined molecular weight and narrow polydispersity (Mw/Mn = 1.09–1.35) were obtained under mild thermal conditions. Linear first-order kinetics with different feeding ratios of AM and cobalt porphyrin were obtained and the number-average molecular weight (Mn) increased linearly with monomer conversion. However, little research has been reported on (TMOP)Co(II) mediating a living radical dispersion polymerization of acrylamide. The effects of the feeding ratio of monomer and cobalt porphyrin, the solvent, the time of polymerization as well as monomer concentration on the morphology of the spherical particles were investigated. There were increasing trends in the particle size with the increasing concentration of monomer, the time of polymerization and feeding ratio of AM and cobalt porphyrin. The effects of the volume ratio of alcohol or DMF and water were also studied.
Introduction
Living radical polymerization (LRP) has been well developed for the synthesis of well-defined polymeric materials with predetermined molecular weight and narrow polydispersity.1 Organometallic mediated radical polymerization (OMRP),2–4 which is one of the LRP methods,5 has also been well-developed. Particularly, cobalt complex mediated radical polymerization (CMRP) represents one of the most successful and efficient OMRP methods.6 Cobalt tetramesitylporphyrin (TMP)Co(II), was the first cobalt complex used to mediate the living radical polymerization of methyl acrylate, and at least 95% of the polymer chains were living even at relatively high conversions.7 When vinyl acetate was used as a monomer, (TMP)Co(II) was also an efficient mediator in living radical polymerization.8,9 Sulfonated cobalt porphyrin complexes (TMPS)Co(II) were used to mediate the LRP of acrylic acid in water. Poly-(acrylic acid) with a high molecular weight (232![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and narrow molecular weight distribution (PDI = 1.20) was rapidly obtained via AA polymerization in the presence of (TMPS)Co(II) and an azo-initiator (V-70).10 Cobalt porphyrin substituted by three mesityl groups and one phenyl group with a long chain alcohol at the para position was designed to increase the solubility in different solvents. This new cobalt porphyrin complex became the first cobalt complex that was capable of controlling the polymerization of acrylamides like N,N-dimethylacrylamide (DMA) and N-isopropylacrylamide (NIPAM).11,12 Polyacrylamide (PAM) is widely used in many fields, owing to its good precipitation properties, for example, as flocculants for wastewater treatment,13–16 retention aids in papermaking,17–19 etc. Traditional polyacrylamide (PAM) prepared via a radical aqueous method has generally been grinded to dry powder for easy storage. However, radical polymerization is generally uncontrolled, that is, the desired molecular weight is difficult to provide. Fortunately, the LRP of acrylamide can be obtained via reversible addition fragmentation transfer (RAFT),20–23 nitroxide mediated radical polymerization (NMP),24–26 atom transfer radical polymerization (ATRP),27–30 and so on. In general, PAM obtained via solution polymerization has a high viscosity and poor solubility.31–33 However, PAM obtained via dispersion polymerization which does not need the grinding process and uniform particles were obtained straight after the emulsion was centrifuged and dried. Nevertheless, only a few OMRPs of acrylamide (or DMA), such as (TMP-OH)Co(II) and (selen)Co(II) mediated living radical polymerization, have been reported.11,12 Specifically, so far, little research has been reported on the LRP of acrylamide in a dispersion system via OMRP.
000) and narrow molecular weight distribution (PDI = 1.20) was rapidly obtained via AA polymerization in the presence of (TMPS)Co(II) and an azo-initiator (V-70).10 Cobalt porphyrin substituted by three mesityl groups and one phenyl group with a long chain alcohol at the para position was designed to increase the solubility in different solvents. This new cobalt porphyrin complex became the first cobalt complex that was capable of controlling the polymerization of acrylamides like N,N-dimethylacrylamide (DMA) and N-isopropylacrylamide (NIPAM).11,12 Polyacrylamide (PAM) is widely used in many fields, owing to its good precipitation properties, for example, as flocculants for wastewater treatment,13–16 retention aids in papermaking,17–19 etc. Traditional polyacrylamide (PAM) prepared via a radical aqueous method has generally been grinded to dry powder for easy storage. However, radical polymerization is generally uncontrolled, that is, the desired molecular weight is difficult to provide. Fortunately, the LRP of acrylamide can be obtained via reversible addition fragmentation transfer (RAFT),20–23 nitroxide mediated radical polymerization (NMP),24–26 atom transfer radical polymerization (ATRP),27–30 and so on. In general, PAM obtained via solution polymerization has a high viscosity and poor solubility.31–33 However, PAM obtained via dispersion polymerization which does not need the grinding process and uniform particles were obtained straight after the emulsion was centrifuged and dried. Nevertheless, only a few OMRPs of acrylamide (or DMA), such as (TMP-OH)Co(II) and (selen)Co(II) mediated living radical polymerization, have been reported.11,12 Specifically, so far, little research has been reported on the LRP of acrylamide in a dispersion system via OMRP.
This article reports that commercially available organometallic derivatives of cobalt tetramethoxyphenylporphyrin, (TMOP)Co(II) (Scheme 1), initiated and controlled the polymerization of acrylamide in a dispersion system to form homopolymers for the first time where the linear increase in number average molecular weight with monomer conversion and relatively small polydispersities (1.05–1.35) were indicative of an effective living radical polymerization process. Furthermore, fair spherical particles of PAM were easily obtained using the dispersion polymerization.
 |
| | Scheme 1 Cobalt porphyrin complex (TMOP)Co(II). | |
Experimental
Materials
Acrylamide (AM, 99%, Tianjin Chemical Reagent First Factory, Tianjin, China), cobalt tetramethoxyphenylporphyrin [(TMOP)Co(II), Alfa Aesar, 98%], polyvinylpyrrolidone (PVP, Mn = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Aladdin Biological and Chemical Reagent Co., Ltd, Shanghai, China) and 2,2′-azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride (VA-044, Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China) were used without further purification. All other reagents were used as received, if not mentioned otherwise.
000, Aladdin Biological and Chemical Reagent Co., Ltd, Shanghai, China) and 2,2′-azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride (VA-044, Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China) were used without further purification. All other reagents were used as received, if not mentioned otherwise.
Preparation of polymer
All of the ingredients such as monomers, PVP (5%), DMF (or alcohol) and deionized water were added to a 100 mL four-necked flask with a stirrer, a reflux condenser, a thermometer, and a nitrogen inlet tube. After purging with nitrogen for 30 min, the polymerization was initiated by adding (TMOP)Co(II) or VA-044 into the system. After specified time intervals, an aliquot was withdrawn and the reaction was stopped by exposure to air, the reaction mixture was stopped at the same time by exposure to air. All of the volatiles in the aliquots were removed and the residue was subjected to monomer conversion determination which was monitored by integration of the monomer vs. polymer methine or methylene resonances in 1H NMR (D2O, 500 MHz). The bulk dispersion system (a picture of the emulsion is shown in Fig. 1a) was centrifuged to remove the solvent, and then washed many times with alcohol. The obtained polymer was further dried in a vacuum oven at 50 °C for 16 h (a picture of the PAM powder is shown in Fig. 1b).
 |
| | Fig. 1 Photographs of PAM. (a) PAM dispersion system; (b) PAM powder obtained by centrifuging the emulsion. | |
Polymer characterization
A 1H NMR spectrum of the polymer was obtained in D2O using a Bruker (500 MHz 1H) NMR spectrometer (Bruker Corporation, Bremen, Germany). The morphologies of the particles in PAM were obtained using a Quanta-250 scanning electron microscope (FEI Corporation, American). The relative molecular weight of the polymer was determined using a Wyatt DAWN Heleos light scattering detector (LSD) at 25 °C. This system comprised a Optilab rEx RI detector, a Shimadzu Waters 2489 UV detector and Shodex OHpak SB-804 HQ and SB-806 HQ (8 × 300 mm) columns mounted in series. The mobile phase consisted of 0.12 mol L−1 NH4(CH3COO) (sodium azide 0.02%) and the flow rate was maintained at 1.0 mL min−1. Poly(ethylene glycol) narrow standards were used to calibrate the LSD using the universal calibration method. The diameters of PAM particles were measured using Image-Pro Plus 6.2 software.34
Results and discussion
It was tested whether cobalt porphyrin (TMOP)Co(II) can initiate and mediate the dispersion polymerization of acrylamide in alcohol/water or DMF/water at 55 °C, as outlined in Table 1. The corresponding polydispersity values remained narrow (Mw/Mn = 1.09–1.35). However, when the monomer to (TMOP)Co(II) ratio was increased to 8911![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the molecular weight obtained in alcohol/water was much smaller than the theoretical value (entries 11 and 12, Table 1), that is to say, the control was poorer in alcohol/water than DMF/water (entries 7 and 9, Table 1), due to the poor solubility of (TMOP)Co(II) in alcohol.
1, the molecular weight obtained in alcohol/water was much smaller than the theoretical value (entries 11 and 12, Table 1), that is to say, the control was poorer in alcohol/water than DMF/water (entries 7 and 9, Table 1), due to the poor solubility of (TMOP)Co(II) in alcohol.
Table 1 Dispersion polymerizations of AM initiated and mediated by (TMOP)Co(II)a
| Run |
[AM]/[Co]/[VA-044] |
Solvent |
Time (min) |
Conv.b (%) |
Mn,thc (×105) |
Mn,GPCd (×105) |
Mw/Mnd |
| [AM]0 = 15%; [Co] = [(TMOP)Co(II)]. The monomer conversion was determined based on 1H NMR spectra (D2O). Mn,th = Mw(AM) × ratio × conv. (%). Determined using gel permeation chromatography in a solution of 0.12 mol L−1 NH4(CH3COO) (sodium azide 0.02%) against poly(ethylene glycol) standards. |
| 1 |
3000/1/0 |
VDMF/VH2O = 7/3 |
60 |
24.81 |
0.954 |
2.295 |
1.20 |
| 2 |
4000/1/0 |
VDMF/VH2O = 7/3 |
20 |
19.35 |
0.992 |
1.610 |
1.23 |
| 3 |
4000/1/0 |
VDMF/VH2O = 7/3 |
30 |
20.63 |
1.058 |
1.966 |
1.21 |
| 4 |
4000/1/0 |
VDMF/VH2O = 7/3 |
50 |
22.48 |
1.153 |
2.839 |
1.16 |
| 5 |
4000/1/0 |
VDMF/VH2O = 7/3 |
70 |
24.24 |
1.243 |
2.902 |
1.16 |
| 6 |
8911/1/0 |
VDMF/VH2O = 7/3 |
30 |
27.5 |
3.141 |
1.152 |
1.35 |
| 7 |
8911/1/0 |
VDMF/VH2O = 7/3 |
60 |
33.3 |
3.803 |
2.911 |
1.09 |
| 8 |
8911/1/0 |
VDMF/VH2O = 7/3 |
90 |
35.9 |
4.100 |
3.703 |
1.28 |
| 9 |
8911/1/5 |
VDMF/VH2O = 7/3 |
30 |
39.7 |
4.534 |
4.253 |
1.26 |
| 10 |
5000/1/0 |
VEtOH/VH2O = 7/3 |
60 |
36.7 |
2.352 |
1.58 |
1.14 |
| 11 |
8911/1/0 |
VEtOH/VH2O = 7/3 |
60 |
45.6 |
5.208 |
1.934 |
1.28 |
| 12 |
8911/1/5 |
VEtOH/VH2O = 7/3 |
40 |
81.2 |
9.274 |
1.691 |
1.33 |
To evaluate the living character of these polymerization processes, the kinetic rate plots for the dispersion polymerization of AM were studied (Fig. 2). The dispersion polymerization of AM initially followed linear first-order kinetics, indicating the generation of a constant concentration of growing radicals during polymerization.35 Different [AM]/[Co] molar ratios ranging from 3000/1 to 8911/1 showed that (TMOP)Co(II) induced LRP of AM in DMF/water could be controlled over a wide range of [AM]/[Co] molar ratios. The apparent rate constant values for the polymerization were determined to be 0.882 × 10−3, 1.37 × 10−3, 2.25 × 10−3 and 2.96 × 10−3 min−1 (corresponding to the 3000/1, 4000/1, 5000/1 and 8911/1 ratios, respectively), indicating that a higher monomer concentration had a faster polymerization rate which is however similar to the results obtained from (salen)Co(II)/TPO.6 It is probable that when the monomer concentration was high, the radicals had more opportunities to react with the monomer and the rate of polymerization increased.36 Take the [AM]/[Co] molar ratios of 4000/1 (Fig. 3a) and 8911/1 (Fig. 3b) as examples, the experimental molecular weight of the PAM increased linearly with monomer conversion. As is shown in Fig. 4, GPC traces for the polymerization of AM took on the form of single peak curves and are reasonably symmetrical. These results demonstrated the living character and controllability of (TMOP)Co(II) in the dispersion polymerization of AM.7 Nevertheless, the deficiencies in this polymerization were very clear. On the one hand, there was a significant discrepancy between the experimental and theoretical molecular weight of PAM (Table 1, entries 2–8). The deviation of the measured and calculated molecular weights results from the system not fulfilling the assumption of complete conversion of cobalt(II) to organocobalt(III) in the solution of DMF/water.11,12,37 On the other hand, the conversion was relatively low since the polymer had already started to coagulate after the conversion reached ∼40% in DMF/water and the reaction had to been terminated. Improving the reaction conditions is currently ongoing to increase the conversion of AM by using other solvents or introducing salt, and so on.
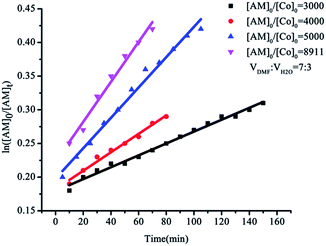 |
| | Fig. 2 Kinetics for the dispersion polymerization of AM with different feeding ratios of AM and (TMOP)Co(II) (conditions: monomer, 15 wt%; VDMF/VH2O = 7/3; temperature, 55 °C). | |
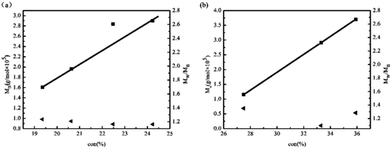 |
| | Fig. 3 Evolution of molar mass and polydispersity versus AM conversion (a) [AM]/[Co] = 4000/1; (b) [AM]/[Co] = 8911/1. | |
 |
| | Fig. 4 GPC traces of PAM: (a) 30 min, Mn = 1.152 × 105; (b) 60 min, Mn = 2.911 × 105; (c) 90 min, Mn = 3.703 × 105 (conditions: [AM]/[Co] = 8911/1; monomer, 15 wt%; VDMF/VH2O = 7/3; temperature, 55 °C). | |
The alcohol or DMF to water ratio (VEtOH/VH2O or VDMF/VH2O) in the dispersion polymerization of AM was also an important parameter which could influence the kinetics and subsequently the morphology of the polymer particles and their properties. In order to determine this effect, the reactions were carried out at alcohol/water ratios of 8/2, 7/3, 6/4 and 5/5, keeping the amount of monomer constant, and the morphology of the particles obtained from these reactions are presented in Fig. 5. The results showed that spherical particles were not obtained at VEtOH/VH2O = 8/2 (Fig. 5a) and VEtOH/VH2O = 6/4 (Fig. 5c), and the particles were not uniform at VEtOH/VH2O = 5/5 (Fig. 5d). Only when the alcohol to water volume ratio was 7/3 (Fig. 5b), could uniform spherical particles be formed. Nevertheless, as shown in Fig. 6, spherical particles could be obtained from the dispersion polymerization of acrylamide over a wider range of volume ratios with DMF/water as the solvent (Fig. 6). When the volume ratio reached VDMF/VH2O = 8/2 and VDMF/VH2O = 7/3, the particle sizes all went into uniformity (Fig. 6a and b). Taking all of these factors into consideration, the morphology of the polymer particles was optimal at VEtOH/VH2O or VDMF/VH2O = 7/3, which was used in the most experiments.
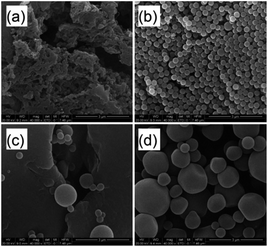 |
| | Fig. 5 Effect of solvent on the morphologies of PAM particles at (a) VEtOH/VH2O = 8/2; (b) VEtOH/VH2O = 7/3; (c) VEtOH/VH2O = 6/4; (d) VEtOH/VH2O = 5/5 (conditions: [AM]/[Co] = 5000/1; monomer, 15 wt%; temperature, 55 °C; time, 60 min). | |
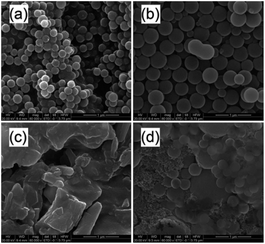 |
| | Fig. 6 Effect of solvent on the morphologies of PAM particles at (a) VDMF/VH2O = 8/2; (b) VDMF/VH2O = 7/3; (c) VDMF/VH2O = 6/4; (d) VDMF/VH2O = 5/5 (conditions: [AM]/[Co] = 5000/1; monomer, 15 wt%; temperature, 55 °C; time, 100 min). | |
Cobalt porphyrin (TMOP)Co(II) was important for achieving a good dispersion system. Without (TMOP)Co(II), the dispersion polymerization of AM was initiated by VA-044 in a solution of VDMF/VH2O = 7/3, however, after 60 min, the polymer with high viscosity had already started to coagulate and stuck to the flask and muddler (Table 2, entry 1). It was probable that the chains of the polymer grew too fast to produce primary particles, then the reaction system formed a block with high viscosity rather than a stable dispersion. Nevertheless, when the dispersion polymerization was conducted under the same conditions mediated by cobalt porphyrin (TMOP)Co(II), a mixture of (TMOP)Co(II) and AM solution in DMF/water for even 80 min still gave a stable dispersion (Table 2, entry 2). Therefore, it is obvious that cobalt porphyrin played a significant role in the dispersion polymerization of AM. The dispersion polymerization of AM was also conducted under the free VA-044 condition and only initiated by (TMOP)Co(II) (Table 2, entries 4–10; Tables 3 and 4). Experiments were carried out to investigate the effect of reaction time, monomer concentration and feeding molar ratio of [AM]/[Co] on particle size in the dispersion polymerization of AM. The data indicated that the particle size increased with the increase in time (Fig. 7 and 8; Table 2, entries 4–10), monomer concentration (Fig. 9; Table 3) and feeding molar ratio of AM and cobalt porphyrin (Fig. 10 and 11; Table 4). With the increase of time, the monomer concentration and feeding molar ratio of [AM]/[Co], the contact probability between the radicals and the monomers increased, which resulted in the high molecular weight, then congregation of primary particles caused the rise in final particle size.
Table 2 Effect of reaction time on PAM particle sizea
| Run |
[AM]/[Co]/[VA-044] |
Solvent |
Time (min) |
Dmean (μm) |
| [AM]0 = 15%; [Co] = [(TMOP)Co(II)]; temperature, 55 °C. |
| 1 |
8911/0/5 |
VDMF/VH2O = 7/3 |
60 |
Coagulated |
| 2 |
8911/1/5 |
VDMF/VH2O = 7/3 |
80 |
0.36 |
| 3 |
8911/1/5 |
VEtOH/VH2O = 7/3 |
40 |
0.96 |
| 4 |
8911/1/0 |
VDMF/VH2O = 7/3 |
10 |
0.19 |
| 5 |
8911/1/0 |
VDMF/VH2O = 7/3 |
60 |
0.26 |
| 6 |
8911/1/0 |
VDMF/VH2O = 7/3 |
120 |
0.37 |
| 7 |
4000/1/0 |
VDMF/VH2O = 7/3 |
30 |
0.19 |
| 8 |
4000/1/0 |
VDMF/VH2O = 7/3 |
60 |
0.23 |
| 9 |
4000/1/0 |
VDMF/VH2O = 7/3 |
70 |
0.26 |
| 10 |
4000/1/0 |
VDMF/VH2O = 7/3 |
120 |
0.33 |
Table 3 Effect of monomer concentration on PAM particle sizea
| Run |
[AM]/[Co]/[VA-044] |
Solvent |
(AM) (wt%) |
Dmean (μm) |
| [Co] = [(TMOP)Co(II)]; time, 60 min; temperature, 55 °C. |
| 1 |
4000/1/0 |
VDMF/VH2O = 7/3 |
15 |
0.23 |
| 2 |
4000/1/0 |
VDMF/VH2O = 7/3 |
17 |
0.26 |
| 3 |
4000/1/0 |
VDMF/VH2O = 7/3 |
18 |
0.28 |
| 4 |
4000/1/0 |
VDMF/VH2O = 7/3 |
20 |
0.34 |
Table 4 Effect of feeding molar ratio on PAM particle sizea
| Run |
[AM]/[Co]/[VA-044] |
Solvent |
(AM) (wt%) |
Dmean (μm) |
| [Co] = [(TMOP)Co(II)]; time, 60 min; temperature, 55 °C. |
| 1 |
3000/1/0 |
VDMF/VH2O = 7/3 |
15 |
0.23 |
| 2 |
4000/1/0 |
VDMF/VH2O = 7/3 |
15 |
0.23 |
| 3 |
5000/1/0 |
VDMF/VH2O = 7/3 |
15 |
0.28 |
| 5 |
7000/1/0 |
VDMF/VH2O = 7/3 |
15 |
0.48 |
| 6 |
4000/1/0 |
VEtOH/VH2O = 7/3 |
15 |
0.27 |
| 7 |
5000/1/0 |
VEtOH/VH2O = 7/3 |
15 |
0.31 |
| 8 |
7911/1/0 |
VEtOH/VH2O = 7/3 |
15 |
0.36 |
| 9 |
8911/1/0 |
VEtOH/VH2O = 7/3 |
15 |
0.43 |
 |
| | Fig. 7 Morphologies of PAM particles at (a) time, 10 min; (b) time, 60 min; (c) time, 120 min (conditions: [AM]/[Co] = 8911/1; monomer, 15 wt%; VDMF/VH2O = 7/3; temperature, 55 °C). | |
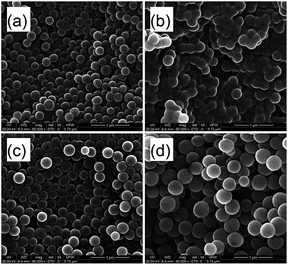 |
| | Fig. 8 Morphologies of PAM particles at (a) time, 30 min; (b) time, 60 min; (c) time, 70 min; (d) time, 120 min. Conditions: [AM]/[Co] = 4000/1; monomer, 15 wt%; VDMF/VH2O = 7/3; temperature, 55 °C. | |
 |
| | Fig. 9 Morphologies of PAM particles at (a) monomer, 15 wt%; (b) monomer, 17 wt%; (c) monomer, 18 wt%; (d) monomer, 20 wt%. Conditions: [AM]/[Co] = 4000/1; VDMF/VH2O = 7/3; temperature, 55 °C; time: 60 min. | |
 |
| | Fig. 10 Morphologies of PAM particles at (a) [AM]/[Co] = 3000/1; (b) [AM]/[Co] = 4000/1; (c) [AM]/[Co] = 5000/1; (d) [AM]/[Co] = 7000/1. Conditions: monomer, 15 wt%; VDMF/VH2O = 7/3; temperature, 55 °C; time: 60 min. | |
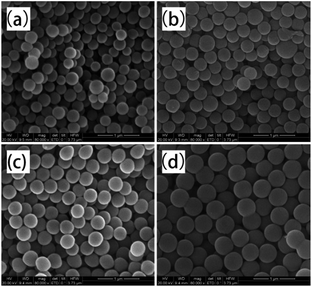 |
| | Fig. 11 Morphologies of PAM particles at (a) [AM]/[Co] = 3000/1; (b) [AM]/[Co] = 4000/1; (c) [AM]/[Co] = 5000/1; (d) [AM]/[Co] = 7000/1. Conditions: monomer, 15 wt%; VEtOH/VH2O = 7/3 temperature, 55 °C; time: 60 min. | |
Conclusions
Cobalt porphyrin (TMOP)Co(II) was successfully used in the dispersion polymerization of AM. The polymerization proceeded via linear kinetics. Polymers with narrow molecular weight distributions and controlled molecular weights were obtained. These results confirmed the living nature of the dispersion polymerization of AM. Furthermore, the dispersion system had good fluidity. Micrographs of the polymers indicated that uniform spherical PAM particles were obtained at VEtOH/VH2O = 7/3 as well as VDMF/VH2O = 8/2 and 7/3. The particle size increased with increasing time, monomer concentration and feeding molar ratio of [AM]/[Co] because of the congregation of primary particles. Work is currently ongoing to combine living radical dispersion polymerization mediated by cobalt porphyrin using other materials as monomers such as acrylates, and so on, to prepare uniform spherical particles.
Acknowledgements
This work was financially supported by the National Science Foundation for Young Scientists of China (No. 21302083 and 51562029), Teaching Reform Project of Inner Mongolia University of Technology (No. 2014212 and 2014217) and the Program of Natural Science Foundation of Inner Mongolia University of Technology, China (No. X201503). The authors thank the Institute of Coal Conversion and Cyclic Economy for their support of these experiments. The authors also thank Professor Xuefeng Fu of Peking University for her helpful discussion.
Notes and references
- W. A. Braunecker and K. Matyjaszewski, Prog. Polym. Sci., 2007, 32, 93–146 CrossRef CAS.
- M. Hurtgen, C. Detrembleur, C. Jerome and A. Debuigne, Polym. Rev., 2011, 51, 188–213 CrossRef CAS.
- L. E. N. Allan, M. R. Perry and M. P. Shaver, Prog. Polym. Sci., 2012, 37, 127–156 CrossRef CAS.
- A. Debuigne, R. Poli, C. Jerome, R. Jerome and C. Detrembleur, Prog. Polym. Sci., 2009, 34, 211–239 CrossRef CAS.
- C.-H. Peng, T.-Y. Yang, Y. Zhao and X. Fu, Org. Biomol. Chem., 2014, 12, 8580–8587 CAS.
- Y. Zhao, S. Zhang, Z. Wu, X. Liu, X. Zhao, C.-H. Peng and X. Fu, Macromolecules, 2015, 48, 5132–5139 CrossRef CAS.
- B. B. Wayland, G. Poszmik, S. L. Mukerjee and M. Fryd, J. Am. Chem. Soc., 1994, 116, 7943–7944 CrossRef CAS.
- C.-H. Peng, J. Scricco, S. Li, M. Fryd and B. B. Wayland, Macromolecules, 2008, 41, 2368–2373 CrossRef CAS.
- C.-S. Hsu, T.-Y. Yang and C.-H. Peng, Polym. Chem., 2014, 5, 3867–3875 RSC.
- C.-H. Peng, M. Fryd and B. B. Wayland, Macromolecules, 2007, 40, 6814–6819 CrossRef CAS.
- Y. Zhao, M. Yu and X. Fu, Chem. Commun., 2013, 49, 5186–5188 RSC.
- Y. Zhao, H. Dong, Y. Li and X. Fu, Chem. Commun., 2012, 48, 3506–3508 RSC.
- Q. Ye, Z. Zhang and X. Ge, J. Appl. Polym. Sci., 2003, 89, 2108–2115 CrossRef CAS.
- J. Zhu, G. Zhang and J. Li, J. Appl. Polym. Sci., 2011, 120, 518–523 CrossRef CAS.
- B. A. Bolto, Prog. Polym. Sci., 1995, 20, 987–1041 CrossRef CAS.
- V. M. Monroy Soto, Polymer, 1984, 25, 254–262 CrossRef.
- T. Sezaki and M. A. Hubbe, Colloids Surf., A, 2006, 289, 89–95 CrossRef CAS.
- D. G. Peiffer, R. D. Lundberg, L. Sedillo and J. C. Newlove, US Pat., 4626285, December 2, 1986.
- S. Lu, R. Liu and X. Sun, J. Appl. Polym. Sci., 2002, 84, 343–350 CrossRef CAS.
- S. Wallyn, Z. Zhang, F. Driessen, J. Pietrasik, B. G. D. Geest, R. Hoogenboom and F. E. D. Prez, Macromol. Rapid Commun., 2014, 35, 405–411 CrossRef CAS PubMed.
- C. Rodriguez-Emmenegger, B. V. K. J. Schmidt, Z. Sedlakova, V. Šubr, A. B. Alles, E. Brynda and C. Barner-Kowollik, Macromol. Rapid Commun., 2011, 32, 958–965 CrossRef CAS PubMed.
- C. Boyer, V. Bulmus, T. P. Davis, V. Ladmiral, J. Liu and S. Perrier, Chem. Rev., 2009, 109, 5402–5436 CrossRef CAS PubMed.
- D. B. Thomas, B. S. Sumerlin, A. B. Lowe and C. L. McCormick, Macromolecules, 2003, 36, 1436–1439 CrossRef CAS.
- C. Magee, Y. Sugihara, P. B. Zetterlund and F. Aldabbagh, Polym. Chem., 2014, 5, 2259–2265 RSC.
- J. Rigolini, B. Grassl, L. Billon, S. Reynaud and O. F. X. Donard, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6919–6931 CrossRef CAS.
- O. Gibbons, W. M. Carroll, F. Aldabbagh, P. B. Zetterlund and B. Yamada, Macromol. Chem. Phys., 2008, 209, 2434–2444 CrossRef CAS.
- G. R. Jones, Z. Li, A. Anastasaki, D. J. Lloyd, P. Wilson, Q. Zhang and D. M. Haddleton, Macromolecules, 2016, 49, 483–489 CrossRef CAS.
- G.-X. Wan, M. Lu, Z.-H. Hou, L.-C. Liu, E.-X. Liang and H. Wu, e-Polym., 2015, 15, 75–79 Search PubMed.
- D. A. Z. Wever, P. Raffa, F. Picchioni and A. A. Broekhuis, Macromolecules, 2012, 45, 4040–4045 CrossRef CAS.
- C. Boyer, N. A. Corrigan, K. Jung, D. Nguyen, T.-K. Nguyen, N. N. M. Adnan, S. Oliver, S. Shanmugam and J. Yeow, Chem. Rev., 2016, 116, 1803–1949 CrossRef CAS PubMed.
- C. L. McCormick and L. C. Salazar, J. Appl. Polym. Sci., 1993, 48, 1115–1120 CrossRef CAS.
- M. J. Fevola, J. K. Bridges, M. G. Kellum, R. D. Hester and C. L. McCormick, J. Appl. Polym. Sci., 2004, 94, 24–39 CrossRef CAS.
- M. J. Fevola, M. G. Kellum, R. D. Hester and C. L. McCormick, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 3252–3270 CrossRef CAS.
- H. Zhu, M. L. Du, M. L. Zou, C. S. Xu, N. Li and Y. Q. Fu, J. Mater. Chem., 2012, 22, 9301–9307 RSC.
- Y. Zhao, M. Yu, S. Zhang, Y. Liu and X. Fu, Macromolecules, 2014, 47, 6238–6245 CrossRef CAS.
- Y. Wu, C. Wang and J. Xu, J. Appl. Polym. Sci., 2010, 115, 1131–1137 CrossRef CAS.
- C.-M. Liao, C.-C. Hsu, F.-S. Wang, B. B. Wayland and C.-H. Peng, Polym. Chem., 2013, 4, 3098–3104 RSC.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and narrow molecular weight distribution (PDI = 1.20) was rapidly obtained via AA polymerization in the presence of (TMPS)Co(II) and an azo-initiator (V-70).10 Cobalt porphyrin substituted by three mesityl groups and one phenyl group with a long chain alcohol at the para position was designed to increase the solubility in different solvents. This new cobalt porphyrin complex became the first cobalt complex that was capable of controlling the polymerization of acrylamides like N,N-dimethylacrylamide (DMA) and N-isopropylacrylamide (NIPAM).11,12 Polyacrylamide (PAM) is widely used in many fields, owing to its good precipitation properties, for example, as flocculants for wastewater treatment,13–16 retention aids in papermaking,17–19 etc. Traditional polyacrylamide (PAM) prepared via a radical aqueous method has generally been grinded to dry powder for easy storage. However, radical polymerization is generally uncontrolled, that is, the desired molecular weight is difficult to provide. Fortunately, the LRP of acrylamide can be obtained via reversible addition fragmentation transfer (RAFT),20–23 nitroxide mediated radical polymerization (NMP),24–26 atom transfer radical polymerization (ATRP),27–30 and so on. In general, PAM obtained via solution polymerization has a high viscosity and poor solubility.31–33 However, PAM obtained via dispersion polymerization which does not need the grinding process and uniform particles were obtained straight after the emulsion was centrifuged and dried. Nevertheless, only a few OMRPs of acrylamide (or DMA), such as (TMP-OH)Co(II) and (selen)Co(II) mediated living radical polymerization, have been reported.11,12 Specifically, so far, little research has been reported on the LRP of acrylamide in a dispersion system via OMRP.
000) and narrow molecular weight distribution (PDI = 1.20) was rapidly obtained via AA polymerization in the presence of (TMPS)Co(II) and an azo-initiator (V-70).10 Cobalt porphyrin substituted by three mesityl groups and one phenyl group with a long chain alcohol at the para position was designed to increase the solubility in different solvents. This new cobalt porphyrin complex became the first cobalt complex that was capable of controlling the polymerization of acrylamides like N,N-dimethylacrylamide (DMA) and N-isopropylacrylamide (NIPAM).11,12 Polyacrylamide (PAM) is widely used in many fields, owing to its good precipitation properties, for example, as flocculants for wastewater treatment,13–16 retention aids in papermaking,17–19 etc. Traditional polyacrylamide (PAM) prepared via a radical aqueous method has generally been grinded to dry powder for easy storage. However, radical polymerization is generally uncontrolled, that is, the desired molecular weight is difficult to provide. Fortunately, the LRP of acrylamide can be obtained via reversible addition fragmentation transfer (RAFT),20–23 nitroxide mediated radical polymerization (NMP),24–26 atom transfer radical polymerization (ATRP),27–30 and so on. In general, PAM obtained via solution polymerization has a high viscosity and poor solubility.31–33 However, PAM obtained via dispersion polymerization which does not need the grinding process and uniform particles were obtained straight after the emulsion was centrifuged and dried. Nevertheless, only a few OMRPs of acrylamide (or DMA), such as (TMP-OH)Co(II) and (selen)Co(II) mediated living radical polymerization, have been reported.11,12 Specifically, so far, little research has been reported on the LRP of acrylamide in a dispersion system via OMRP.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Aladdin Biological and Chemical Reagent Co., Ltd, Shanghai, China) and 2,2′-azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride (VA-044, Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China) were used without further purification. All other reagents were used as received, if not mentioned otherwise.
000, Aladdin Biological and Chemical Reagent Co., Ltd, Shanghai, China) and 2,2′-azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride (VA-044, Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China) were used without further purification. All other reagents were used as received, if not mentioned otherwise.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the molecular weight obtained in alcohol/water was much smaller than the theoretical value (entries 11 and 12, Table 1), that is to say, the control was poorer in alcohol/water than DMF/water (entries 7 and 9, Table 1), due to the poor solubility of (TMOP)Co(II) in alcohol.
1, the molecular weight obtained in alcohol/water was much smaller than the theoretical value (entries 11 and 12, Table 1), that is to say, the control was poorer in alcohol/water than DMF/water (entries 7 and 9, Table 1), due to the poor solubility of (TMOP)Co(II) in alcohol.











