Fabrication and ionic transportation characterization of funnel-shaped nanochannels†
Abstract
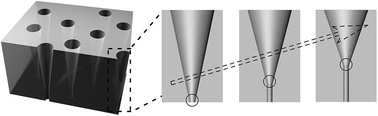
Solid-state nanochannels have attracted increasing interest because they exhibit similar properties to, but are more stable than, biological nanopores. However, existing solid-state nanochannels, such as conical-shaped nanochannels, possess a limited critical region at the tip side, and thus demonstrate poor controllability of the orientation and magnitude of ionic rectification. In this work, we demonstrate the fabrication of funnel-shaped nanochannels with a gradual structural transformation. The longer critical regions can be controlled by tuning the etching temperature. In addition, the rectification ratio can be increased from about 3 to 6 by introducing a longer critical region. Using finite-element analysis based on the Poisson–Nernst–Planck (PNP) equations, it can be found that the increased length of the critical region could contribute to the asymmetric ion transportation. The funnel-shaped nanochannels with enhanced asymmetric ion transportation may find applications in the fields of materials, electronics, and life sciences.

 Please wait while we load your content...
Please wait while we load your content...