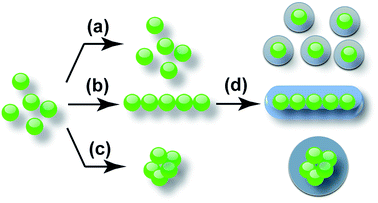
Chainlike assembly of oleic acid-capped NaYF4:Yb,Er nanoparticles and their fixing by silica encapsulation†
Junfei Xue‡
ab,
Junwei Zhao‡b,
Jian Wub,
Pengyu Xuab,
Sheng Chenab,
Yaping Dinga and
Weihai Ni*b
aDepartment of Chemistry, College of Sciences, Shanghai University, Shanghai, 200444, China
bDivision of i-Lab & Key Laboratory for Nano-Bio Interface Research, Suzhou Institute of Nano-Tech & Nano-Bionics, Chinese Academy of Sciences, Suzhou, Jiangsu 215123, China. E-mail: whni2012@sinano.ac.cn
First published on 22nd June 2016
Abstract
We demonstrate, through simply tuning the polarity of the dispersant system, that oleic acid (OA)-capped NaYF4 nanoparticles can be self-assembled into chainlike structures. The assembled chainlike structures were further fixed by silica encapsulation via the Stöber method. The reported approach can be readily extended to other OA-capped nanoparticles in organic solvents.
Rare earth-doped nanoparticles exhibit unique upconversion (UC) luminescence with large anti-Stokes shift, narrow emission band, and superior photostability.1 Thanks to these excellent properties, UC nanoparticles have been widely used in many applications, such as biological labelling and imaging,2 display technology,3 solar cells,4 photocatalysis,5,6 solid-state lasers,7 etc. Up to now, a variety of chemical approaches have been developed toward the synthesis of UC nanoparticles, which includes coprecipitation, thermal decomposition, hydro(solvo)thermal, and other methods.8 The thermal decomposition method developed by Yan et al. was firstly used to fabricate highly monodisperse LaF3 nanoparticles.9 After years of development, the method has been used as a common strategy to synthesize high quality NaYF4 nanoparticles, where oleic acid (OA) was usually chosen as the coordinating ligand.10–12 As an efficient host matrix for the doping of rare earth ions, NaYF4 have attracted a great amount of attention in recent years.13,14 In particular, Yb3+ and Er3+ co-doped NaYF4 nanoparticles have been successfully applied in detection of biomolecules.15
The properties of assembled nanoparticles are largely different from individual ones due to close interactions between the adjacent nanoparticles. In fact, many applications are based on the assembled structures rather than individuals. These assemblies with ordered structures can be formed by means of solvent evaporation,16 electric dipolar interaction,17 electrostatic interactions,18 magnetic dipolar interaction,19,20 van der Waals forces,21 hydrogen bonds,22 and etc., which are driven by the change of the surroundings. That is, the self-assembly can be triggered by some particular stimuli from the environment. Usual stimuli include temperature, light, pH, or solvent polarity, and these parameters can be used to control the assembly via direct intermolecular interactions between capping ligands of the surfaces.23,24 2-D or 3-D assemblies of UC nanoparticles have been realized and investigated previously.25 In comparison with 2-D or 3-D ones, 1-D assemblies allow for the coupling of electronic, magnetic, or optical properties along a specific direction. Moreover, from a microscopic point of view, nanoparticles in a chain with well-defined structures (e.g. two or more nanoparticles in a row) are of particular significance as ideal objects in the investigation on light–matter interactions. Nevertheless, 1-D chainlike assemblies require more specific conditions for the interactions between nanoparticles. For example, formation of 1-D nanocrystal chains were aided by introducing molecular clusters.26 Citrate-stabilized gold nanoparticles can form chainlike assembly in aqueous18,27 and ethanol28 solutions, which is driven by electrostatic interactions. In analogy to magnetic dipolar systems,20,29,30 electric dipolar interaction is believed to be responsible for the growing of semiconductor nanodots and nanocrystals of several nanometers into nanorods or nanowires.17,31,32 Yan et al. found that the composition of dispersant can greatly influence the formation of the assembly of RE2O3 nanodisks, which was assisted by the facets of the nanodisks.33 For spherical dielectric nanoparticles with diameters of tens of nanometers, however, their assembly into chainlike structures remains challenging because they possess no facets and encounter larger van der Waals forces usually ending up with disordered aggregation.
Herein, we demonstrate, through simply tuning the polarity of the dispersant system, quasi-spherical OA-capped NaYF4 nanoparticles can be efficiently self-assembled into chainlike structures. This approach can be readily extended to other OA-capped nanoparticles. As shown in Scheme 1, the nanoparticles are monodispersed in pure cyclohexane (CHX) (a), while their assembly can be triggered by adding ethanol (EtOH) into CHX. Finely tuning the EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHX ratio at 1
CHX ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (b) leads to the formation of chainlike assembled structures, which is supported by a statistical analysis on the collinearity of the chain. Clustered nanoparticles appear at EtOH
6 (b) leads to the formation of chainlike assembled structures, which is supported by a statistical analysis on the collinearity of the chain. Clustered nanoparticles appear at EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHX of 1
CHX of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (c), and aggregates are found in pure EtOH. Discrete size distribution of the assembled structures was observed, which is dependent on the composition of the dispersant. The chainlike assembly is the consequence of the dipole–dipole interaction between the nanoparticles at a certain condition achieved by tuning the polarity of the dispersant system. The assembled chainlike structures are further fixed by silica encapsulation via the Stöber method (d). In comparison to those before the silica encapsulation, the chainlike structures with the silica shell do not show noticeable degrading or disruption of the order of arrangement in the chain, clearly indicating the formation of the chainlike assembly in the solution phase as well as strong attraction between the particles. The assembled structures with the silica shell are readily redispersable in a variety of solutions without any structural change and can be preserved for a long time.
2 (c), and aggregates are found in pure EtOH. Discrete size distribution of the assembled structures was observed, which is dependent on the composition of the dispersant. The chainlike assembly is the consequence of the dipole–dipole interaction between the nanoparticles at a certain condition achieved by tuning the polarity of the dispersant system. The assembled chainlike structures are further fixed by silica encapsulation via the Stöber method (d). In comparison to those before the silica encapsulation, the chainlike structures with the silica shell do not show noticeable degrading or disruption of the order of arrangement in the chain, clearly indicating the formation of the chainlike assembly in the solution phase as well as strong attraction between the particles. The assembled structures with the silica shell are readily redispersable in a variety of solutions without any structural change and can be preserved for a long time.
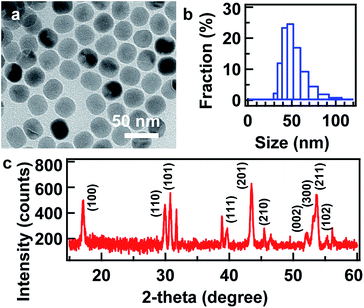
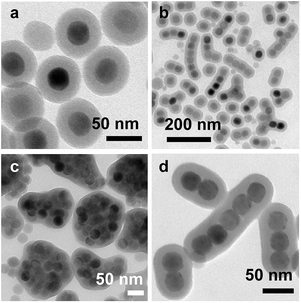
As aforementioned, NaYF4 is an efficient host matrix material for the doping of rare earth ions. The rare earth-doped NaYF4 nanoparticles with hexagonal phase exhibit UC luminescence ∼10 times stronger than those with cubic phase.13 Therefore, hexagonal NaYF4 was chosen to be the ideal candidate for the assembly in this study. NaYF4:Yb,Er nanoparticles were synthesized using the thermal decomposition method.12 OA and ODE were used in the synthesis, which are essential for obtaining NaYF4 nanoparticles with high quality. ODE was used as the primary solvent, and OA was employed as the capping ligand to protect the as-synthesized nanoparticles from aggregation. Their quantities were finely tuned to control the size and shape of the resultant NaYF4 nanoparticles. The as-synthesized NaYF4:Yb,Er nanoparticles are uniform quasi-spherical particles with a statistically average size of 33.8 ± 1.7 nm from TEM measurements (Fig. 1a). It is consistent with most probable size of around 43.8 nm based on the DLS measurement (Fig. 1b), where the ligand shell is considered. XRD patterns obtained from the NaYF4:Yb,Er nanoparticles matches well with the standard hexagonal NaYF4 (JCPDS card no. 16-0334), suggesting high crystallinity of the nanoparticles (Fig. 1c). Upon laser irradiation at 978 nm, the NaYF4:Yb,Er nanoparticles dispersed in CHX exhibit emission bands peaked at 525, 540, and 655 nm, corresponding to the 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transitions, respectively (Fig. S1a, ESI†). The emission band at 540 nm is much stronger than the other two, resulting in the green appearance of the UC nanoparticle powder (Fig. S1b, ESI†).
The as-synthesized nanoparticles are capped with OA. To remove excess ligands, the nanoparticles were precipitated by adding acetone, followed by centrifugation, washed with a mixture of EtOH and CHX (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
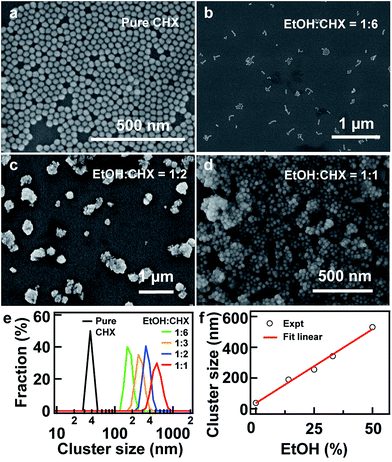
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) three times, and redispersed in CHX at a reduced concentration. The resultant nanoparticles were monodispersed in pure CHX, which were confirmed by SEM (Fig. 2a). To initializing the assembly, a calculated amount of EtOH was added to the suspension of the nanoparticles. The volume ratio of EtOH
1 v/v) three times, and redispersed in CHX at a reduced concentration. The resultant nanoparticles were monodispersed in pure CHX, which were confirmed by SEM (Fig. 2a). To initializing the assembly, a calculated amount of EtOH was added to the suspension of the nanoparticles. The volume ratio of EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHX was controlled at 1
CHX was controlled at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. The morphologies of the nanoparticles were examined by SEM. Chainlike assembly of the nanoparticles was obtained at 1
1, respectively. The morphologies of the nanoparticles were examined by SEM. Chainlike assembly of the nanoparticles was obtained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 of the EtOH
6 of the EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHX ratio (Fig. 2b). With the increase of the volume ratio, the assembly clusters includes more nanoparticles. For instance, at 1
CHX ratio (Fig. 2b). With the increase of the volume ratio, the assembly clusters includes more nanoparticles. For instance, at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of EtOH
2 of EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHX the size of the clusters becomes much bigger and the shape gets irregular (Fig. 2c). Large aggregates of the nanoparticles was observed at 1
CHX the size of the clusters becomes much bigger and the shape gets irregular (Fig. 2c). Large aggregates of the nanoparticles was observed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of EtOH
1 of EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHX (Fig. 2d). The nanoparticles were severely aggregated in pure EtOH (Fig. S2, ESI†). This trend can be clearly revealed by the DLS measurement (Fig. 2e). The peaked size increases from 43.8 to 190.1, 255, 342, and 531.2 nm with increasing EtOH volume. Moreover, discrete peaks with small overlaps are shown in the size distribution. It suggests that the assembling process is very efficient and involves most of the nanoparticles, which can also be confirmed by few isolated nanoparticles found in the SEM images (Fig. 2b and c).
CHX (Fig. 2d). The nanoparticles were severely aggregated in pure EtOH (Fig. S2, ESI†). This trend can be clearly revealed by the DLS measurement (Fig. 2e). The peaked size increases from 43.8 to 190.1, 255, 342, and 531.2 nm with increasing EtOH volume. Moreover, discrete peaks with small overlaps are shown in the size distribution. It suggests that the assembling process is very efficient and involves most of the nanoparticles, which can also be confirmed by few isolated nanoparticles found in the SEM images (Fig. 2b and c).
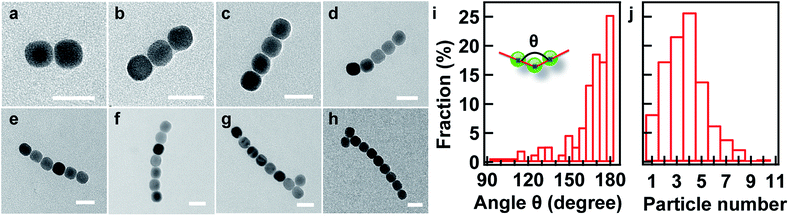
To provide quantitative information on chainlike structures, a statistical analysis was performed to investigate the collinearity of the chain. The assembled samples were first examined by TEM, where various structures can be identified. Fig. 3a–h show typical nanoparticle structures obtained from the assembly consisting of 2–9 nanoparticles in a chain, respectively, where the structures close to collinearity can be visually observed. Angle θ was introduced, which was defined as the angle between the lines connecting the centers of three consecutive nanoparticles in the chain (inset, Fig. 3i). It is shown that the angle in the range from 150° to 180° occurs in more than 90% of the chain structures. The statistical measurement also includes irregular chainlike assemblies (Fig. S3, ESI†). This result indicates that the formation of a chainlike structure is regulated by the oriented attachment mechanism, and it is a highly probable behaviour under the specific condition. Interestingly, a preferential bending is observable in the chainlike structures (Fig. 3b–h), which further supports proposed mechanism on the formation of the chain.
The chainlike-forming nanoparticles can be categorized into semiconductor, dielectric, and metal ones. The dipole–dipole attraction was considered to be the most probable driving force that induces semiconductor or dielectric nanoparticles of TiO2, ZnO, Y2O3, or CdTe to assemble into chainlike nanostructures through oriented attachment mechanism.17,31,32,34 The dipole moment is permanent35 and strong as a general attribute to all semiconductor or dielectric nanoparticles.34 Surface localized charges are believed to be responsible for the large permanent dipole moments present in these nanoparticles.35 In metal nanoparticles, however, the charges can be easily delocalized, and hence they cannot display such dipole moment. Instead, the chainlike assembly of metal nanoparticles is attributed to electrostatic interactions. The dielectric nanoparticles studied in our work are tens of nanometers in size, which is one order of magnitude larger than the nanodots studied in the previous works. Here we employed the theoretical description for the estimation of the magnitude of the attraction. It is known from the previous report that the dipole moment of a 5.6 nm nanodot is about 98 D.17 If the linear size-dependence35 is still applicable and valid for large particles, the dipole moment of a 34 nm nanoparticle can be as large as 600 D. The energy of dipole attraction can be written as: E = μ2/2πε0r(r2 − dNP2),17 where μ is the dipole moment of the nanoparticle, r is the center-to-center interdipolar separation, and dNP is the particle diameter. Considering dNP = 34 nm and r = 35 nm in our case, the calculation gives the attraction energy equal to 18 kJ mol−1. This value is two times larger than 8.8 kJ mol−1, calculated for a 3.4 nm quantum dot with μ = 50 D.17 This result indicates that the magnitude of the attraction generally increases with the increase of the particle size, providing the possibility of the dominant role of the dipolar attraction at specific conditions.
Dispersant composition greatly affects the status of the surface ligands as well as the formation of self-organized patterns.33 The alkyl chain possessed by OA is hydrophobic, and therefore the nanoparticles can be well dispersed in a nonpolar solvent such as CHX. The mutual short-range repulsive forces between the ligands prevent the nanoparticles from aggregation and keep them monodispersed. Addition of a polar solvent (e.g., EtOH) increases the polarity of the dispersant as well as the interfacial tension, leading to partial immersion of the alkyl chains and compaction of the ligands in the solvent. As a result, the repulsive force provides by the ligands between the particles is weakened, and the nanoparticles begin to get closer to each other. In the meanwhile, the dipole–dipole attractive force becomes important. The attachment of nanoparticles is regulated by the dipole–dipole interaction, which is orientation preferable, leading to the chainlike assembly. Further increase of the polarity of the dispersant resulted in the stable attachment of nanoparticles from all the directions and irregular clusters are formed, where the dipolar interaction is overwhelmed by the van der Waals interactions. When the particles are dispersed in a polar solvent, the alkyl chains could not be immersed in the solvent any more, and the nanoparticles would severely aggregate.
The self-assembly process is irreversible. Once the assemblies are formed, they cannot be disassembled simply by changing the polarity of the dispersant back to the original. This is confirmed by a control experiment characterizing the morphology of the products after redispersing the chainlike assemblies into pure CHX. As shown in the SEM image (Fig. S4, ESI†), the chainlike structures remain almost unchanged after redispersing in CHX. On the basis of the fact, the chain formation is believed to be a kinetic process. Therefore, increasing the polarity of the dispersant actually accelerates the formation of the chain. A statistical analysis was performed on 462 chainlike assembled structures formed at EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHX = 1
CHX = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. Fig. 3j shows that the statistical histogram of the particle number in the chain is peaked at about 4, indicating the efficient formation of the chainlike structures at the appropriate polarity of the dispersant. By controlling the polarity of the dispersant, one can actually tune the size of the assembled structure as well as the chain length. This proposed mechanism is also clearly evidenced by the observed linear dependence of the cluster size on the volume percentage of the EtOH in the solution mixture (Fig. 2f).
6. Fig. 3j shows that the statistical histogram of the particle number in the chain is peaked at about 4, indicating the efficient formation of the chainlike structures at the appropriate polarity of the dispersant. By controlling the polarity of the dispersant, one can actually tune the size of the assembled structure as well as the chain length. This proposed mechanism is also clearly evidenced by the observed linear dependence of the cluster size on the volume percentage of the EtOH in the solution mixture (Fig. 2f).
The TEM and SEM images evidenced the success of realizing the chainlike assembly of the nanoparticles. However, formation of the assembled structures during the evaporation of the solvents is also possible, though their precipitation from the solution to the TEM grids or substrates was performed in a controlled manner. To exclude such possibility, we adopted the approach of silica encapsulation proposed by Xia and co-workers36 and apply it in our system, which is expected to provide direct evidence on the solution-phase formation of the chainlike assemblies. Through this approach, we also expect that the chainlike assembled structures can be fixed by the silica encapsulation and redispersable into a variety of solutions without any structural change. The silica encapsulation was performed following a modified Stöber method reported previously.37 The assembled nanoparticles were first washed twice to remove EtOH residues from the solution. Note that the ultrasound-assisted dispersion during the washing needs to be done gently and rapidly to avoid disruption of the assembly. The silica encapsulation was performed respectively on isolated, chainlike-assembled, and clustered nanoparticles shown in Fig. 2a–c through the condensation of TEOS in CHX. We noticed that the appearance of the solution samples remained unchanged after the silica encapsulation. Fig. 4a–c show the TEM images of the corresponding resultant products. The thickness of the silica shell is about 15 nm. In comparison to those before the silica encapsulation, the chainlike structures with the silica shell do not show noticeable degrading or disruption of the order of arrangement in the chain (Fig. 4d), clearly indicating the formation of the chainlike assembly in the solution phase instead of on the TEM grids or silicon substrates during the solvent evaporation. Moreover, the dipolar interaction-driven assemblies are proved so robust that most of them can survive under the harsh conditions of the silica encapsulation, exhibiting a high yield (Fig. S5, ESI†). In comparison with other stabilization approaches such as crosslinking,38,39 the silica encapsulation provide more stable structures for preserving the chainlike assemblies. The assembly is achieved at the equilibrium of the steric repulsion and dipolar attraction, which is susceptible under some external stimuli, such as ultrasonication. After the silica coating, the structures can be treated as rigid entities like legumes. Actually, our method provides another possibility for making the filaments.
In conclusion, we demonstrate, through simply tuning the polarity of the dispersant system, OA-capped NaYF4 nanoparticles can be self-assembled into chainlike structures. Finely tuning the EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHX ratio at 1
CHX ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 leads to the efficient formation of chainlike assembled structures, which is supported by a statistical analysis on the collinearity of the chain. The chainlike assembly is the consequence of the dipole–dipole interaction between the nanoparticles at a certain condition achieved by tuning the polarity of the dispersant system. The assembled chainlike structures are further fixed by silica encapsulation via the Stöber method. The assembled structures with the silica shell are readily redispersable in a variety of solutions without any structural change and can be preserved for a long time. This approach can be readily extended to other OA-capped nanoparticles.
6 leads to the efficient formation of chainlike assembled structures, which is supported by a statistical analysis on the collinearity of the chain. The chainlike assembly is the consequence of the dipole–dipole interaction between the nanoparticles at a certain condition achieved by tuning the polarity of the dispersant system. The assembled chainlike structures are further fixed by silica encapsulation via the Stöber method. The assembled structures with the silica shell are readily redispersable in a variety of solutions without any structural change and can be preserved for a long time. This approach can be readily extended to other OA-capped nanoparticles.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grants 21271181 and 21473240) and Thousand Youth Talent Program of China.References
- X. Y. Chen, Y. S. Liu and D. T. Tu, Lanthanide-doped luminescent nanomaterials: from fundamentals to bioapplications, Springer-Verlag Press, Berlin, 2014 Search PubMed.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976 RSC.
- E. Downing, L. Hesselink, J. Ralston and R. Macfarlane, Science, 1996, 273, 1185 CrossRef CAS.
- X. Huang, S. Han, W. Huang and X. Liu, Chem. Soc. Rev., 2013, 42, 173 RSC.
- J.-H. Kim and J.-H. Kim, J. Am. Chem. Soc., 2012, 134, 17478 CrossRef CAS PubMed.
- Z. X. Li, F. B. Shi, T. Zhang, H. S. Wu, L. D. Sun and C. H. Yan, Chem. Commun., 2011, 47, 8109 RSC.
- F. Heine, E. Heumann, T. Danger, T. Schweizer, G. Huber and B. Chai, Appl. Phys. Lett., 1994, 65, 383 CrossRef CAS.
- H. Dong, S. R. Du, X. Y. Zheng, G. M. Lyu, L. D. Sun, L. D. Li, P. Z. Zhang, C. Zhang and C. H. Yan, Chem. Rev., 2015, 115, 10725 CrossRef CAS PubMed.
- Y.-W. Zhang, X. Sun, R. Si, L.-P. You and C.-H. Yan, J. Am. Chem. Soc., 2005, 127, 3260 CrossRef CAS PubMed.
- H. X. Mai, Y. W. Zhang, R. Si, Z. G. Yan, L. D. Sun, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2006, 128, 6426 CrossRef CAS PubMed.
- J. C. Boyer, F. Vetrone, L. A. Cuccia and J. A. Capobianco, J. Am. Chem. Soc., 2006, 128, 7444 CrossRef CAS PubMed.
- Z. Q. Li and Y. Zhang, Nanotechnology, 2008, 19, 345606 CrossRef PubMed.
- S. Heer, K. Kömpe, H. U. Güdel and M. Haase, Adv. Mater., 2004, 16, 2102 CrossRef CAS.
- K. W. Krämer, D. Biner, G. Frei, H. U. Güdel, M. P. Hehlen and S. R. Lüthi, Chem. Mater., 2004, 16, 1244 CrossRef.
- L. Wang, R. Yan, Z. Huo, L. Wang, J. Zeng, J. Bao, X. Wang, Q. Peng and Y. Li, Angew. Chem., Int. Ed., 2005, 44, 6054 CrossRef CAS PubMed.
- E. Rabani, D. R. Reichman, P. L. Geissler and L. E. Brus, Nature, 2003, 426, 271 CrossRef CAS PubMed.
- Z. Tang, N. A. Kotov and M. Giersig, Science, 2002, 297, 237 CrossRef CAS PubMed.
- H. Zhang and D. Y. Wang, Angew. Chem., Int. Ed., 2008, 47, 3984 CrossRef CAS PubMed.
- J. Sun, Y. Zhang, Z. Chen, J. Zhou and N. Gu, Angew. Chem., Int. Ed. Engl., 2007, 46, 4767 CrossRef CAS PubMed.
- M. Klokkenburg, R. P. Dullens, W. K. Kegel, B. H. Erne and A. P. Philipse, Phys. Rev. Lett., 2006, 96, 037203 CrossRef PubMed.
- B. L. V. Prasad, S. I. Stoeva, C. M. Sorensen and K. J. Klabunde, Langmuir, 2002, 18, 7515 CrossRef CAS.
- W. H. Ni, R. A. Mosquera, J. Perez-Juste and L. M. Liz-Marzan, J. Phys. Chem. Lett., 2010, 1, 1181 CrossRef CAS.
- M. Grzelczak, J. Vermant, E. M. Furst and L. M. Liz-Marzán, ACS Nano, 2010, 4, 3591 CrossRef CAS PubMed.
- K. J. M. Bishop, C. E. Wilmer, S. Soh and B. A. Grzybowski, Small, 2009, 5, 1600 CrossRef CAS PubMed.
- W. Feng, L. D. Sun, Y. W. Zhang and C. H. Yan, Coord. Chem. Rev., 2010, 254, 1038 CrossRef CAS.
- X. Zhang, L. Lv, G. Guo, L. Liu, D. Han, B. Wang, Y. Tu, J. Hu, D. Yang and A. Dong, J. Am. Chem. Soc., 2016, 138, 3290 CrossRef CAS PubMed.
- E. C. Cho, S. W. Choi, P. H. Camargo and Y. Xia, Langmuir, 2010, 26, 10005 CrossRef CAS PubMed.
- X. Han, J. Goebl, Z. Lu and Y. Yin, Langmuir, 2011, 27, 5282 CrossRef CAS PubMed.
- P. De Gennes and P. Pincus, Phys. Kondens. Mater., 1970, 11, 189 CrossRef CAS.
- T. Hao, Adv. Mater., 2001, 13, 1847 CrossRef CAS.
- C. Pacholski, A. Kornowski and H. Weller, Angew. Chem., Int. Ed., 2002, 41, 1188 CrossRef CAS PubMed.
- Y. W. Jun, M. F. Casula, J. H. Sim, S. Y. Kim, J. Cheon and A. P. Alivisatos, J. Am. Chem. Soc., 2003, 125, 15981 CrossRef CAS PubMed.
- R. Si, Y.-W. Zhang, H.-P. Zhou, L.-D. Sun and C.-H. Yan, Chem. Mater., 2007, 19, 18 CrossRef CAS.
- Y. Zhang, J. Guo, T. White, T. T. Y. Tan and R. Xu, J. Phys. Chem. C, 2007, 111, 7893 CrossRef CAS.
- M. Shim and P. Guyot-Sionnest, J. Chem. Phys., 1999, 111, 6955 CrossRef CAS.
- E. C. Cho, S.-W. Choi, P. H. C. Camargo and Y. Xia, Langmuir, 2010, 26, 10005 CrossRef CAS PubMed.
- H. S. Qian, H. C. Guo, P. C. Ho, R. Mahendran and Y. Zhang, Small, 2009, 5, 2285 CrossRef CAS PubMed.
- R. Dreyfus, J. Baudry, M. L. Roper, M. Fermigier, H. A. Stone and J. Bibette, Nature, 2005, 437, 862 CrossRef CAS PubMed.
- Y. Xiong, Q. Chen, N. Tao, J. Ye, Y. Tang, J. Feng and X. Gu, Nanotechnology, 2007, 18, 345301 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional SEM and TEM images, the luminescence image and spectrum of UC nanoparticles. See DOI: 10.1039/c6ra09545c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |