Injectable SVF-loaded porcine extracellular matrix powders for adipose tissue engineering
Yongzhou Lu†
a,
Chuanlong Jia†a,
Bo Bib,
Liang Chena,
Yiqun Zhoua,
Ping Yanga,
Yu Guoa,
Jingjing Zhua,
Ningwen Zhuc and
Tianyi Liu*a
aDepartment of Plastic and Reconstructive Surgery, Hua Dong Hospital, Fu Dan University, No. 221 West Yan'an Road, 200041 Shanghai, China. E-mail: drluther@163.com; Tel: +86 13162877696
bClinical Laboratory, Hua Dong Hospital, Fu Dan University, Shanghai, China
cDepartment of Dermatology, Hua Shan Hospital, Fu Dan University, Shanghai, China
First published on 26th May 2016
Abstract
This study provides a novel method in injectable tissue engineering which contains porcine extracellular matrix (ECM) powder scaffolds and stromal-vascular fraction (SVF) cells. We fabricated ECM powders from porcine adipose tissue and mixed the SVF cell suspension together with the ECM powders immediately after the isolation. SVF-loaded ECM powders and their controls were respectively injected into the dorsa of mice. After 6 weeks, the interest sites were harvested and analyzed using histology, immunohistochemistry, and quantitative-polymerase chain reaction (qPCR). Histology and immunohistochemistry demonstrated that the presence of the SVF cells loaded in the ECM powders promoted adipogenesis, angiopoiesis and matrix remodeling. This was in parallel with upregulation of host cell recruitment and adipogenesis genes. The results provide cellular and molecular evidence suggesting a novel method in injectable tissue engineering. We believe that SVF-loaded ECM powders could act as efficient injectable biomaterials for tissue engineering and have great potential for meeting clinical challenges in regenerative medicine, particularly in relation to adipose tissue engineering.
1. Introduction
Injectable tissue engineering scaffolds are widely used in cosmetic and reconstruction surgery not only because they minimize the risk of infections, scarring, and high costs, but also because they may be used to restore body contours in patients who have lost contour on account of surgical resections, trauma, or congenital abnormalities.1,2 The injectable scaffolds are fabricated either from natural materials, including collagen, chitosan, and alginate, or from synthetic materials, including poly(glycolide) (PGA), poly(lactide) (PLA), poly(caprolactone) (PCL), and poly(γ-benzyl-L-glutamate) (PBLG).3,4 Injectable scaffolds are an ideal choice for adipose tissue engineering due to the simple implantation technique and the ability to fill irregular defects.5Scaffolds in tissue engineering strategies should be designed to include biological and chemical cues that mimic the native microenvironment.6 Cells are supported by the intricate extracellular matrix (ECM) in vivo, which contains collagen, elastin, laminin, fibronectin, and glycosaminoglycans (GAGs).7 ECM helps hold cells together in tissues, and performs protective and supportive functions. Furthermore, the ECM influences cellular behavior and responses, such as survival, development, shape, migration, and polarity, by interacting with cellular adhesion molecules, growth regulators, binding proteins, proteolytic enzymes, and enzyme inhibitors.8 ECM scaffolds which are widely used in clinics for regeneration of various tissues and organs have been harvested from various tissues including small intestinal submucosa (SIS),9 urinary bladder,10 cholecyst,11 blood vessels,12 heart valves,13 skin,14 liver,15 and adipose tissue.16 The widespread use of ECM scaffolds across many clinical applications is attributed to their excellent biocompatibility, biodegradability, and inductive properties.
Adipose tissue not only contains various ECM components, but also contains many endocrine and paracrine factors commonly referred to as adipokines. Moreover, adipose tissue is the most prevalent, expendable, and safely harvested tissue in human body. Seeding ECM scaffolds with regenerative cell populations and creating tissue substitutes that can be used to generate predictable and stable adipose tissue is a major strategy in adipose tissue engineering.17 Adipose-derived stem cells (ASCs) are a readily and ideal cell source for adipose tissue engineering because of their sufficient availability, minimally invasive procurement, high proliferation and adipogenic differentiation potential.18 Human adipose ECM powders scaffolds seeded with ASCs have been extensively studied as xenografts to reconstruct adipose tissue.2,16 However, the ethical implication invoked and the difficulty for mass production of the ECM powder derived from human adipose tissue has impeded its further application. In fact, harvesting autologous fat tissues may be impossible in certain population of patients, such as lipodystrophy complicated to HIV and cachexia following chemotherapy.19–21 Additionally, the requirement of a two stage procedure for allograft will increase the risk of infection and the length of hospital stay.
Here we seek to investigate the ECM powders derived from porcine subcutaneous fat tissue and its ability as a scaffold to facilitate adipose differentiation. Pigs have been widely used in the fields of biological materials and several clinical settings. The ECM-based scaffolds derived from decellularized porcine urinary bladders and SIS as xenografts to reconstruct musculoskeletal structures, cardiovascular tissues, and skin have been extensively investigated.9,22,23 A number of porcine decellularized xenogenic products are now being introduced into the market and have received regulatory approval for use in human using tissues from porcine SIS, porcine urinary bladder, and porcine heart valves.24,25 A major advantage of ECM powders derived from porcine adipose tissue is the possibility of mass production due to the readily available abundant fat tissue and relatively simple harvest technique. It also facilitates clinical application without ethical challenge.
Stromal-vascular fraction (SVF) cells including smooth muscle cells, endothelial cells, fibroblasts, blood cells, and ASCs are mixture cellular components contained in adipose tissue.26,27 SVF cells can be easily isolated from adipose tissue by simple physical treatments, thereby circumventing the need of culturing of ASCs.28 By injecting SVF-loaded ECM carriers in vivo and evaluating the adipogenic differentiation of the grafts, we found that ECM powders scaffolds seeded with SVF cells were able to form adipose tissue. The presence of SVF was essential in modulating the expression of adipogenic factors. In addition, we showed that host cells play a crucial role in the adipose tissue regeneration.
2. Materials and methods
Our study involves the use of live animals including pigs, rabbits, and nude mice. All our experiments were performed in compliance with the relevant laws and institutional guidelines. And our experimental protocol was consented by Animal Care and Use Committee of Fudan university (Shanghai, China).2.1. Fabrication of porcine ECM powders from adipose tissue
Porcine subcutaneous fat tissue was obtained from healthy domesticated pigs between 6 and 12 months of age which had been killed at the slaughterhouse. The adipose tissue obtained from swine was cut into pieces and washed several times with distilled water to remove blood components. The adipose tissue fragments was squeezed into mushy (60 mL). Distilled water (60 mL) was added to the pasty adipose tissue and the tissue/water mixture was homogenized at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min at room temperature using a homogenizer (JJ-2B, China). The tissue suspension was oscillated in the shaking table at 200 rpm for 3 min at room temperature and the upper oil-containing layer was discarded. This process was repeated several times. The gel-like, thick tissue suspension (20 mL) was washed several times by addition of distilled water (20 mL) to the gel-like tissue suspension, gentle mixing by pipetting, and centrifugation at 3000 rpm for 3 min. The final gel-like tissue precipitate was frozen at −80 °C, freeze-dried, and crushed using the homogenizer. The freeze-dried fragments was grinded into powders in a glass mortar.
000 rpm for 5 min at room temperature using a homogenizer (JJ-2B, China). The tissue suspension was oscillated in the shaking table at 200 rpm for 3 min at room temperature and the upper oil-containing layer was discarded. This process was repeated several times. The gel-like, thick tissue suspension (20 mL) was washed several times by addition of distilled water (20 mL) to the gel-like tissue suspension, gentle mixing by pipetting, and centrifugation at 3000 rpm for 3 min. The final gel-like tissue precipitate was frozen at −80 °C, freeze-dried, and crushed using the homogenizer. The freeze-dried fragments was grinded into powders in a glass mortar.
2.2. Isolation of rabbit's adipose-derived SVF cells from adipose tissue
SVF cells were isolated from subcutaneous fat tissue in the groin area of healthy New Zealand White rabbits. The adipose tissue was cut into pieces and washed with phosphate-buffered saline (PBS) containing 5% penicillin/streptomycin (P/S). SVF cells were isolated by digestion with 1.25 mg mL−1 collagenase NB4 (SERVA, German) in DMEM supplemented with 10% fetal bovine serum (Gibco, Australia) and 1% P/S in the shaking table at 200 rpm for 1 h at 37 °C. The resulting cell suspension was centrifuged at 3000 rpm for 3 min and the upper layer was discarded. Red blood cell lysis buffer (RCLB) was added to the precipitate for 10 min at room temperature. After removal of red blood cells, the digested tissue was filtered through a 40 μm strainer to remove aggregated tissue and debris. The filtered suspension was centrifuged at 1000 rpm for 3 min and the upper layer was discarded. The SVF cells was suspended in DMEM at 105 cells per mL.2.3. Scanning electron microscopy (SEM)
The structures of porcine ECM powders were observed using scanning electron microscopy (SEM, Hitachi S-4800 FE-SEM, Japan). The porcine ECM powders were fixed to metal stubs and coated with platinum by sputtering at an accelerating voltage of 15 kV.2.4. In vivo experiments
Eighteen female mice (BALB/c-nu, 6–8 weeks old) were randomly divided into three groups that received differently designed biomaterial implants or cells. The following groups of designed implantation materials or cells were injected into the dorsum of the mice using an 18-gauge needle: 0.2 mL ECM mixed with 0.2 mL SVF cells suspension (ECM + SVF group), 0.2 mL ECM mixed with 0.2 mL DMEM (ECM group), 0.2 mL SVF cells suspension (SVF group). During the study, the mice were bred under a constant laminar airflow and given standard laboratory chow and water. In the sixth week after injections, the skin flaps including the implantation site were removed and fixed.2.5. Histological analysis
Specimens for paraffin embedding were fixed with 4% paraformaldehyde, dehydrated through graded ethanol solutions, cleared in xylene, and embedded in paraffin. Samples were sectioned at 5 μm thickness and stained with H&E or Masson-trichrome. Morphologic alterations were examined by light microscopy and documented by photographs.2.6. Immunohistochemical analysis
The paraffin section of implants were stained for CD31 immunohistochemistry. Briefly, the sections were dewaxed and rehydrated, and then incubated in 3% hydrogen peroxide in methanol solution for 25 min to block endogenous peroxidase activity in the tissue. The primary antibody of mouse anti-rabbit for CD31 (GB13063, Goodbio, China) was added at a concentration of 10 mg mL−1. After incubation overnight (4 °C), the slides were rinsed with PBS for three times and then incubated with the HRP-conjugated rabbit anti-goat antibody (GB22204, DAKO, Denmark), according to the manufacturer's instructions, without dilution at 37 °C for 50 min. Then, the sections were reacted with diaminobenzidine tetrahydrochloride (DAKO, Denmark). Finally, the slides were counterstained with hematoxylin, dehydrated through graded alcohols, and mounted with rhamsan gum.2.7. Quantitative real-time polymerase chain reaction (qPCR)
RNA extraction from the skin flap including the implantation site was performed using TRIzol (Invitrogen, USA) reagent according to the manufacturer's instructions. Prior the extraction, all samples were homogenized. After the samples were measured to ensure similar volume. RNA concentration was determined by measuring the optical absorbance of the samples at 260 nm. The extracted RNA sample (2 μg) was initially reverse transcribed for first strand cDNA synthesis using a PrimeScript 1st Strand cDNA synthesis kit (TaKaRa, China). The reactions were performed in a T3 thermocycler (Biometra, Germany). Real-time PCR was performed using a quantitative real-time amplification system (ABI PRISM 7500 Fast Real-time PCR System; Applied Biosystems, Foster City, CA, USA). SYBR Premix Ex Taq (Tli RNaseH Plus) (Takara, China) was used in each reaction. To minimize confounders we performed PCR of each gene from all three groups of sample (ECM + SVF group, ECM group and SVF group) at the same batch. A housekeeping gene beta-actin was used as an internal control to correct differences in the amount of RNA in each sample. The target genes analyzed in the samples were: murine peroxisome proliferating activated receptor γ (PPARγ), adipocyte fatty acid-binding protein (aP2), adiponectin, leptin, and rabbit PPARγ, aP2, adiponectin, leptin. The primers used for genes of interest were showed in the Table 1. Each experiment was performed in triplicate to minimize errors. The relative expression level of each gene of interest was calculated by normalizing the quantified cDNA transcript level (cycle threshold) to that of beta-actin using the ABI PRISM 7500 Fast Real-Time PCR System.| Gene | Origin | Forward and reverse primer sequences | Gene bank accession no. | Primer size (bp) | Product size (bp) | Melting temperature (°C) | Annealing temperature (°C) |
|---|---|---|---|---|---|---|---|
| aP2 | Mouse | 5′ CCT TTC TCA CCT GGA AGA CA 3′ | M0514f | 20 | 95 | 81.9 | 60 |
| Mouse | 5′CGA AGT TTT CAC TGG AGA CA 3′ | M0514r | 20 | ||||
| Rabbit | 5′CCA GTG AGA ACT TTG ACG ATT AC 3′ | M0511f | 23 | 113 | 83.6 | 60 | |
| Rabbit | 5′GAT CAC ATC CCC ATT CAC aA 3′ | M0511r | 19 | ||||
| Adiponectin | Mouse | 5′ATG TAC CCA TTC GCT TTA CTA 3′ | M0515f | 21 | 120 | 81.3 | 60 |
| Mouse | 5′ACA CCG TGA TGT GGT AAG aA 3′ | M0515r | 19 | ||||
| Rabbit | 5′GTG CCC ATT CGC TTT ACT aA 3′ | M0512f | 19 | 128 | 83 | 60 | |
| Rabbit | 5′TCC TTC ATA TAG ACG GTG ATG T 3′ | M0512r | 22 | ||||
| Leptin | Mouse | 5′ACT CCA CAA TGC TTG ACT CA 3′ | M0516f | 20 | 117 | 83.8 | 60 |
| Mouse | 5′GAT GGG ATG GCT CTT ATC TC 3′ | M0516r | 20 | ||||
| Rabbit | 5′GCC CTG TCT GTC CTG TGT T 3′ | M0513f | 19 | 108 | 84.7 | 60 | |
| Rabbit | 5′TGC GTG TGT GAG ATG TCA CT 3′ | M0513r | 20 | ||||
| PPAR-r | Mouse | 5′CAC TCG CAT TCC TTT GAC AT 3′ | M0094f | 20 | 155 | 81.8 | 60 |
| Mouse | 5′TTG ATC GCA CTT TGG TAT TCT 3′ | M0094r | 21 | ||||
| Rabbit | 5′ATG GTT GCG GAT TAC AAG TAT 3′ | M0510f | 21 | 127 | 81.5 | 60 | |
| Rabbit | 5′AAG GCT CTT CGT GAG TCT TAT 3′ | M0510r | 21 | ||||
| β-actin | Mouse | 5′CCT CTA TGC CAA CAC AGT 3′ | tnt0409f | 18 | 155 | 86.1 | 60 |
| Mouse | 5′AGC CAC CAA TCC ACA CAG 3′ | tnt0409r | 18 | ||||
| Rabbit | 5′GCA GAA ACG AGA CGA GAT TG 3′ | M0132f | 20 | 167 | 80.8 | 60 | |
| Rabbit | 5′GCA GAA CTT TGG GGA CTT TG 3′ | M0132r | 20 |
3. Results
3.1. Fabrication of the ECM and SVF
The porcine ECM powders were prepared using simple physical treatments without the addition of any chemicals or enzymatic factors. We obtained an adipose-derived matrix that retains properties from adipose tissue. The lipids were removed and the ECM remained as a white fibrous tissue scaffold. The product was freeze-dried and then ground to powders by milling (Fig. 1). The ECM is porous in nature, facilitating cell migration and nutrient diffusion. SEM images of the materials show both rugged and smooth surfaces of the ECM powders (Fig. 2). These various structures appeared to be suitable for adhesion and proliferation of cells. SVF cells suspension was mixed together with the ECM powders immediately after the isolation. | ||
| Fig. 2 The SEM micrographs show the rough (left) and the smooth (right) surfaces of porcine ECM powders. | ||
3.2. Macroscopic observations
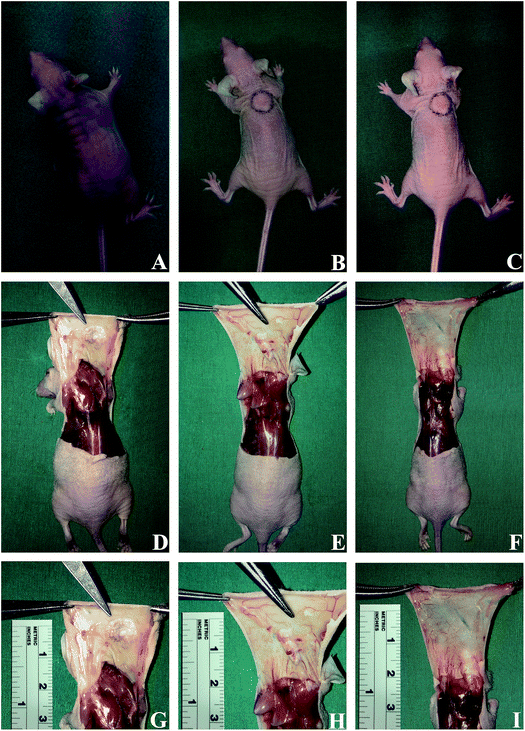
To assess the biocompatibility, mechanical stability, cell ingrowth and adipogenic differentiation in vivo, a suspension of ECM powders with SVF cells was subcutaneously injected into nude mice. As controls, ECM powders without SVF cells and SVF cells alone were also subcutaneously injected into nude mice. The injected porcine ECM powders were easily identified throughout the experimental period of six weeks, as shown in Fig. 3. However, the injected suspension containing SVF cells could not be identified after six weeks. The porcine ECM powders adhered to surrounding tissues and the surface of grafts revealed new vessel formation. The graft of SVF-loaded porcine ECM powders was larger in volume than other grafts.3.3. Histology and immunohistochemistry
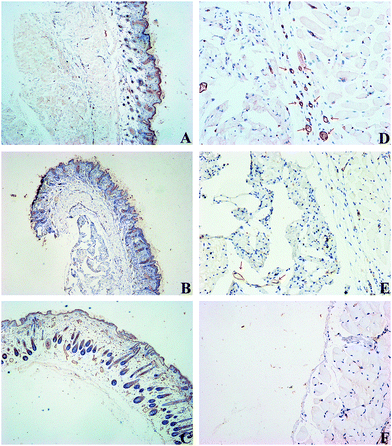
The skin flaps including grafts were histologically examined by H&E and Masson-trichrome staining (Fig. 4 and Fig. 5). Both grafts of SVF-loaded porcine ECM powders and porcine ECM powders alone showed new tissue without any signs of tissue necrosis, cystic spaces, or fibrosis. However, the images of SVF cells group showed nothing under the skin. ECM powders stained strongly for collagen (blue). The collagen fibers of SVF-loaded porcine ECM powders was much more well-organized than their counterparts suggesting that the addition of SVF cells could have led to matrix remodelling. For immunostaining, cells in the matrix stained positive for CD31, indicating vascular development, which is the necessary prerequisite for formation of new adipose tissue (Fig. 6). Overall the neovascularization, ingrowth of mouse cells from the neighboring host tissue, and new tissue formation were more prominent in the grafts of SVF-loaded porcine ECM powders than other groups. Additionally, the ECM powders alone could induce the formation of new blood vessels and ingrowth of mouse cells.3.4. Gene expression of adipogenic markers
The expression of adipogenic genes such as PPARγ, aP2, adiponectin, and leptin was evaluated by qPCR. SVF group and fresh rabbit's skin containing subcutaneous tissues were depicted for comparison.The results above suggest that the pre-seeded SVF cells could significantly induce adipogenesis of host tissue. Thus, qPCR analysis of the grafts suggested the ingrowth, migration and adipogenic differentiation of mouse cells from neighboring host tissues. Additionally, porcine ECM powders alone could slightly induce adipogenesis of host tissue.
4. Discussion
ECM scaffolds consist of the structural and functional molecules secreted by the resident cells of each tissue and organ from which they are prepared.29,30 Therefore, the specific composition and distribution of the ECM constituents will vary depending on the tissue source. We confirm that the ECM powders scaffold derived from porcine adipose tissue is a biological scaffold material for subcutaneous fat tissue engineering. A logistical aspect to consider when trying to translate a tissue engineering technology towards a clinical application is the time required to produce the substitute that will be implanted. The method presented in this paper combines the advantages of both scaffold-based tissue engineering and the self-assembly approach. In our study, ASCs was replaced by SVF cells. SVF cells can be easily isolated from adipose tissue by simple treatments, thereby circumventing the need of culturing of ASCs. Therefore, we can produce SVF-loaded ECM powders materials and inject the mixture to the area of interest in one operation avoiding the long waiting of the ASCs culture and the risks of a second surgery.31 Our study provide a novel method in injectable tissue engineering which is never published before. We believe that our method enables the fabrication of an SVF-containing ECM powders media construct presenting adipogenic property similar to or better than those of adipose tissue construct fabricated using the previously reported method, while reducing the production time by one to two weeks. Moreover, the SVF cells seeded on the ECM powders can induce the adipogenesis of host tissue without having to harvest from rabbits, paving the way for autologous SVF cells graft after seeding on the acellular ECM powders.The forepassed experimental study investigated structures and properties of the human ECM powders scaffolds.32–34 The human ECM powders can pass through an 18-gauge needle. Human ECM powders and accompanying cells can be injected directly into cavities of any size or shape.16,32 However, the ethical implication invoked and the difficulty for mass production of the ECM powder derived from human adipose tissue has impeded its further application. In fact, harvesting autologous fat tissues may be impossible in certain population of patients, such as lipodystrophy complicated to HIV and cachexia following chemotherapy. Additionally, the requirement of a two stage procedure for allograft will increase the risk of infection and the length of hospital stay. In our study, we fabricated the ECM powders from porcine subcutaneous adipose tissue. The biological and morphological characteristics of porcine ECM powders are similar to human ECM powders. The in vivo results provide evidence that porcine ECM powders support in vivo adipogenesis in combination with SVF cells. The SVF cells alone transplantation has yielded poor results, with nothing remaining in the injected area. ECM powders with SVF cells exhibited not only an increased graft volume, but also the formation of new adipose tissue with numerous blood vessels in the grafts. The in vivo engraftment of engineered fat mostly dependent on in-growth of host derived blood vessels. The angiogenesis in neo-generated tissues was abundant, indicating sufficient blood supply for engineered fat survival. As expected, prominent adipogenesis was observed in the ECM powders initially seeded with the SVF cells. Notably, we observed that adipogenesis was proceeding in grafts of porcine ECM powders alone. This result suggests that ECM powders induce ingrowth and differentiation of cells such as preadipocytes from the neighboring host tissues. Furthermore, we discovered that the collagen fibers in ECM + SVF group were much more well-organized than their counterparts. This result suggests that the addition of SVF cells could have led to matrix remodelling. Although the exact mechanisms of how SVF cells remodeled the matrix are not clear, many studies have documented the ability of adipocytes to secrete various paracrine factors, which may promote the degradation of collagen fractions and the formation of well-organized collagen fibers.35–37
During adipogenesis progression, several adipogenesis-related genes involved in adipocyte differentiation have been identified. These factors act cooperatively in adipogenic differentiation by activating the expression of one another, regulating the expression of other adipocyte-specific genes critical to adipogenesis, lipid metabolism, and lipid uptake, and therefore inducing fat cell differentiation.17 PPARγ is an essential regulator of adipocyte differentiation and lipid storage, and lipid metabolism.38 aP2 is a key regulator of intracellular transport and metabolism of fatty acids and a predominant fatty acid-binding protein in adipose tissue. The expression of aP2 is almost exclusively confined to fat tissue and adipogenic cell lines, and highly regulated during adipocyte differentiation. Adiponectin is expressed in a differentiation-dependent fashion in adipocytes and is critical genes in the regulation of biological and metabolic processes in these cells, and it is highly induced during differentiation.39 The present study demonstrates that leptin stimulates lipolysis, and decreases fatty acid uptake in adipocytes, supporting an anti-adipogenic role for leptin, and that leptin expression and release increased from preadipocytes to mature adipocytes in culture.40 Thus, the upregulation of these markers activity in engineered adipose tissue is a clear indication of a physiologically relevant progression toward adipogenesis. We injected rabbit SVF cells and ECM powders into nude mice in order to explore the source of neo-generated adipose cells. In our study, the results suggested that the ingrowth, migration and adipogenic differentiation of cells were from neighboring host mouse tissues. However, the adipogenesis genes of rabbit cells can not be detected. For ECM group, we injected ECM powders without rabbit cells into mice, and there was no doubt that we can not detect rabbit adipogenic genes during six weeks. The ECM influences cellular behavior and responses, such as survival, development, shape, migration, and polarity, by interacting with cellular adhesion molecules, growth regulators, binding proteins, proteolytic enzymes, and enzyme inhibitors. For SVF group, the SVF cells could not survival without appropriate external environment and the rabbit adipogenic genes could not be detected during six weeks. ECM helps hold cells together in tissues, and performs protective and supportive functions. ECM powders scaffolds supported an ideal growing environment to cells. If SVF cells could differentiate into adipose cells in the scaffolds we would detect rabbit adipogenic genes during six weeks. In fact, the rabbit adipogenic genes were not detected in the sixth week after injection. We can confirm that the rabbit's adipogenic markers could not be detected not only in the sixth week but also before six weeks. We speculated that the SVF cells themselves could not differentiate into adipose cells but they could secretion a lot of factors to induce the host cells' adipogenesis.
One major limitation in this study was the absence of the immunogenicity test. Although, some studies have confirmed the low immunogenicity of acellular ECM powders.41 Further studies will be required to investigate the effect of SVF-loaded ECM powders in rats with normal immune system. The latter strategy can be employed in parallel with histological and histomorphometric scoring of the degree of ECM powders degradation or the relationship between the number of grafted SVF cells and the volume of neo-generated adipose tissue, over a prolonged period of time.
5. Conclusion
We fabricated ECM powders from porcine adipose tissue for injectable tissue engineering. The ECM powders prepared in this study provide suitable structure and biocompatibility for seeded SVF cells proliferation and adipogenic differentiation. The SVF-loaded ECM powders exhibited the ability to promote host cell infiltration and angiogenesis. Additionally, the collagen fibers in ECM + SVF group were much more well-organized than their counterparts. Our findings demonstrate that the ECM powders scaffolds seeded with SVF cells have the ability to structure adipose tissue, and the presence of SVF cells modulate the expression of factors crucial for adipogenesis. Furthermore, the injectable SVF-loaded ECM powders allow easy manipulation and minimally invasive surgical procedures in adipose tissue engineering, focusing on the potential clinical application of this approach for soft tissue augmentation and contour improvement.Acknowledgements
This work was supported by the National Natural Science Foundation of China (81272125 and 81301642), the Program of Shanghai Medicine Subject Chief Scientist (XBR2011033), and the National High Technology Research and Development Program (“863” Program) of China (SS2014AA020705).References
- F. Chen, S. Yu, B. Liu, Y. Ni and C. Yu, et al., An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering, Sci. Rep., 2016, 6, 20014 CrossRef CAS PubMed.
- W. Sun, J. Fang, Q. Yong, S. Li, Q. Xie and J. Yin, et al., Subcutaneous construction of engineered adipose tissue with fat lobule-like structure using injectable poly-benzyl-L-glutamate microspheres loaded with adipose-derived stem cells, PLoS One, 2015, 10(8), e0135611 Search PubMed.
- D. B. Khadka and D. T. Haynie, Protein- and peptide-based electrospun nanofibers in medical biomaterials, Nanomedicine, 2012, 8(8), 1242–1262 CAS.
- J. Qian, X. Yong, W. Xu and X. Jin, Preparation and characterization of bimodal porous poly(gamma-benzyl-L-glutamate) scaffolds for bone tissue engineering, Mater. Sci. Eng., C, 2013, 33(8), 4587–4593 CrossRef CAS PubMed.
- R. Yao, R. Zhang, F. Lin and J. Luan, Injectable cell/hydrogel microspheres induce the formation of fat lobule like microtissues and vascularized adipose tissue regeneration, Biofabrication, 2012, 4(4), 045003 CrossRef PubMed.
- M. P. Lutolf and J. A. Hubbell, Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering, Nat. Biotechnol., 2005, 23, 47–55 CrossRef CAS PubMed.
- J. Tien and C. M. Nelson, Microstructured extracellular matrices in tissue engineering and development: an update, Ann. Biomed. Eng., 2014, 42(7), 1413–1423 CrossRef PubMed.
- P. Friedl, K. S. Zänker and E. B. Bröcker, Cell migration strategies in 3-D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function, Microsc. Res. Tech., 1998, 43(5), 369–378 CrossRef CAS PubMed.
- J. H. Spiegel and T. J. Egan, Porcine small intestine submucosa for soft tissue augmentation, Dermatol. Surg., 2004, 30, 1486–1490 Search PubMed.
- H. Yuan, Y. Zhuang, J. Xiong, W. Zhi and L. Liu, et al., Human umbilical mesenchymal stem cells-seeded bladder acellular matrix grafts for reconstruction of bladder defects in a canine model, PLoS One, 2013, 8(11), e80959 Search PubMed.
- K. Burugapalli, A. Thapasimuttu, J. C. Chan, L. Yao and S. Brody, et al., Scaffold with a natural mesh-like architecture: isolation, structural, and in vitro characterization, Biomacromolecules, 2007, 8(3), 928–936 CrossRef CAS PubMed.
- J. Bourget, R. Gauvin, D. Larouche, A. Lavoie and R. Labbé, et al., Human fibroblast-derived ECM as a scaffold for vascular tissue engineering, Biomaterials, 2012, 33(36), 9205–9213 CrossRef CAS PubMed.
- E. Rieder, G. Seebacher, M. T. Kasimir, E. Eichmair and B. Winter, et al., Tissue engineering of heart valves: decellularized porcine and human valve scaffolds differ importantly in residual potential to attract monocytic cells, Circulation, 2005, 111(21), 2792–2797 CrossRef PubMed.
- T. M. MacLeod, P. Sarathchandra, G. Williams, R. Sanders and C. J. Green, Evaluation of a porcine origin acellular dermal matrix and small intestinal submucosa as dermal replacements in preventing secondary skin graft contraction, Burns, 2004, 30(5), 431–437 CrossRef CAS PubMed.
- P. Lin, W. C. Chan, S. F. Badylak and S. N. Bhatia, Assessing porcine liver-derived biomatrix for hepatic tissue engineering, Tissue Eng., 2004, 10(7–8), 1046–1053 CrossRef CAS PubMed.
- J. S. Choi, H. J. Yang, B. S. Kim, J. D. Kim and J. Y. Kim, et al., Human extracellular matrix (ECM) powders for injectable cell delivery and adipose tissue engineering, J. Controlled Release, 2009, 139(1), 2–7 CrossRef CAS PubMed.
- J. R. Mauney, T. Nguyen, K. Gillen, C. Kirker-Head and J. M. Gimble, et al., Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds, Biomaterials, 2007, 28(35), 5280–5290 CrossRef CAS PubMed.
- Y. S. Choi, S. M. Cha, Y. Y. Lee, S. W. Kwon and C. J. Park, et al., Adipogenic differentiation of adipose tissue derived adult stem cells in nude mouse, Biochem. Biophys. Res. Commun., 2006, 345(2), 631–637 CrossRef CAS PubMed.
- D. C. Wan, A. T. Lim and M. T. Longaker, Craniofacial autologous fat transfer, J. Craniofac. Surg., 2009, 20(2), 273–274 CrossRef PubMed.
- P. G. Cordeiro, Breast reconstruction after surgery for breast cancer, N. Engl. J. Med., 2008, 359(15), 1590–1601 CrossRef CAS PubMed.
- G. D. Rosson, M. Magarakis, S. M. Shridharani, S. M. Stapleton and L. K. Jacobs, et al., A review of the surgical management of breast cancer: plastic reconstructive techniques and timing implications, Ann. Surg. Oncol., 2010, 17(7), 1890–1900 CrossRef PubMed.
- K. A. Robinson, J. Li, M. Mathison, A. Redkar and J. Cui, et al., Extracellular matrix scaffold for cardiac repair, Circulation, 2005, 112, I135–I143 CrossRef PubMed.
- S. Y. Chun, G. J. Lim, T. G. Kwon, E. K. Kwak and B. W. Kim, et al., Identification and characterization of bioactive factors in bladder submucosa matrix, Biomaterials, 2007, 28, 4251–4256 CrossRef CAS PubMed.
- J. J. Glynn, E. G. Polsin and M. T. Hinds, Crosslinking decreases the hemocompatibility of decellularized, porcine small intestinal submucosa, Acta Biomater., 2015, 14, 96–103 CrossRef CAS PubMed.
- J. Zhou, B. Nie, Z. Zhu, J. Ding and W. Yang, et al., Promoting endothelialization on decellularized porcine aortic valve by immobilizing branched polyethylene glycolmodified with cyclic-RGD peptide: an in vitro study, Biomed. Mater., 2015, 10(6), 065014 CrossRef PubMed.
- J. M. Bliley, L. Satish, M. M. McLaughlin, R. E. Kling and J. R. Day, et al., Imaging the stromal vascular fraction during soft-tissue reconstruction, Plast. Reconstr. Surg., 2015, 136(6), 1205–1215 CrossRef CAS PubMed.
- L. H. Mangum, J. A. Crow, J. V. Stokes, G. E. Howell 3rd and M. K. Ross, et al., Exposure to p,p′-DDE alters macrophage reactivity and increases macrophage numbers in adipose stromal vascular fraction, Toxicol. Sci., 2016, 150(1), 169–177 CrossRef PubMed.
- G. Rigotti, L. Charles-de-Sá, N. F. Gontijo-de-Amorim, C. M. Takiya and P. R. Amable, et al., Expanded stem cells, stromal-vascular fraction, and platelet-rich plasma enriched fat: comparing results of different facial rejuvenation approaches in a clinical trial, Aesthetic Surg. J., 2016, 36(3), 261–270 CrossRef PubMed.
- B. A. Aguado, J. R. Caffe, D. Nanavati, S. S. Rao and G. G. Bushnell, et al., Extracellular matrix mediators of metastatic cell colonization characterized using scaffold mimics of the pre-metastatic niche, Acta Biomater., 2016, 33, 13–24 CrossRef CAS PubMed.
- A. Turri, I. Elgali, F. Vazirisani, A. Johansson and L. Emanuelsson, et al., Guided bone regeneration is promoted by the molecular events in the membrane compartment, Biomaterials, 2016, 84, 167–183 CrossRef CAS PubMed.
- H. Orbay, Y. Takami, H. Hyakusoku and H. Mizuno, Acellular dermal matrix seeded with adipose-derived stem cells as a subcutaneous implant, Aesthetic Plast. Surg., 2011, 35(5), 756–763 CrossRef PubMed.
- I. Wu, Z. Nahas, K. A. Kimmerling, G. D. Rosson and J. H. Elisseeff, An injectable adipose matrix for soft-tissue reconstruction, Plast. Reconstr. Surg., 2012, 129(6), 1247–1257 CrossRef CAS PubMed.
- S. F. Badylak, D. O. Freytes and T. W. Gilbert, Extracellular matrix as a biological scaffold material: structure and function, Acta Biomater., 2009, 5(1), 1–13 CrossRef CAS PubMed.
- D. A. Young, D. O. Ibrahim, D. Hu and K. L. Christman, Injectable hydrogel scaffold from decellularized human lipoaspirate, Acta Biomater., 2011, 7(3), 1040–1049 CrossRef CAS PubMed.
- R. K. Schneider, J. Anraths, R. Kramann, J. Bornemann and M. Bovi, et al., The role of biomaterials in the direction of mesenchymal stem cell properties and extracellular matrix remodelling in dermal tissue engineering, Biomaterials, 2010, 31(31), 7948–7959 CrossRef CAS PubMed.
- H. Eto, H. Suga, D. Matsumoto, K. Inoue and N. Aoi, et al., Characterization of structure and cellular components of aspirated and excised adipose tissue, Plast. Reconstr. Surg., 2009, 124(4), 1087–1097 CrossRef CAS PubMed.
- C. Lequeux, G. Oni, C. Wong, O. Damour and R. Rohrich, et al., Subcutaneous fat tissue engineering using autologous adipose-derived stem cells seeded onto a collagen scaffold, Plast. Reconstr. Surg., 2012, 130(6), 1208–1217 CrossRef CAS PubMed.
- M. J. Den Broeder, V. A. Kopylova, L. M. Kamminga and J. Legler, Zebrafish as a model to study the role of peroxisome proliferating activated receptors in adipogenesis and obesity, PPAR Res., 2015, 1–11 CrossRef PubMed.
- J. Samulin, S. Lien, E. Grindflek, I. Berget and B. Ruyter, et al., Depot specific differences during adipogenesis of porcine stromal-vascular cells, Cell Biol. Int., 2008, 32(5), 525–531 CrossRef CAS PubMed.
- C. Salmerón, M. Johansson, M. Asaad, A. R. Angotzi and I. Rønnestad, et al., Roles of leptin and ghrelin in adipogenesis and lipid metabolism of rainbow trout adipocytes in vitro, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2015, 188, 40–48 CrossRef PubMed.
- J. S. Choi, B. S. Kim, J. Y. Kim, J. D. Kim and Y. C. Choi, et al., Decellularized extracellular matrix derived from human adipose tissue as a potential scaffold for allograft tissue engineering, J. Biomed. Mater. Res., Part A, 2011, 97(3), 292–299 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |