Nano-engineering of liposomes using a supercritical CO2 mediated gas anti-solvent method†
Abstract
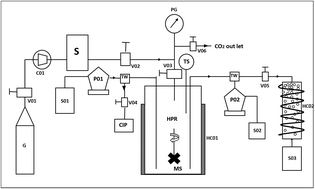
Nano-liposomes were designed using a supercritical (SC) gas anti-solvent (GAS) method. The present approach was configured to combine two steps, i.e., (1) the SC-CO2 assisted drying of phospholipids to form a uniform layer; and (2) the hydration under pressure and depressurization of the SC liquid phase to generate nano-liposomes. The effects of pressure, temperature and surfactant concentration (Tween 80) were evaluated for the production of nano-liposomes. The SC-GAS method was compared with Bangham's method in terms of the mean diameter, coefficient of uniformity, morphology, stability index and actual energy required for liposome formation. Liposomes obtained using Bangham's method were multi-lamellar vesicles, having a coefficient of uniformity of 12.07 ± 0.024 with a mean diameter of 4.64 ± 0.010 μm, exhibiting the irregular thickness of the bilayer. Liposome production using the SC-GAS method under optimized formulation conditions, i.e., 170 bar, 50 °C, 1.5% Tween 80 and a depressurization rate of 25 bar min−1, yielded nano-liposomes exhibiting a coefficient of uniformity of 1.10 ± 0.012 with a mean diameter of 0.063 ± 0.002 μm. The stability index and viscosity of the prepared liposome suspension indicate greater stability and near Newtonian behaviour respectively. The SC-GAS process requires 1.52 times less energy compared to Bangham's method to achieve nano-scale liposome production. Storage studies indicate that SC-GAS based nano-liposomes were highly stable for up to 3 months at different temperatures.

 Please wait while we load your content...
Please wait while we load your content...