High catalytic activity of Co3O4 nanoparticles encapsulated in a graphene supported carbon matrix for oxygen reduction reaction†
Guiting Xieab,
Bohong Chenb,
Zhongqing Jiangc,
Xiaojun Niu*a,
Si Chengb,
Zihao Zhenb,
Yu Jiangb,
Haibo Rongb and
Zhong-Jie Jiang *b
*b
aCollege of Environment and Energy, South China University of Technology, Guangzhou 510006, China. E-mail: xjniu@scut.edu.cn
bNew Energy Research Institute, College of Environment and Energy, South China University of Technology, Guangzhou 510006, China. E-mail: zhongjiejiang1978@hotmail.com; eszjiang@scut.edu.cn
cDepartment of Chemical Engineering, Ningbo University of Technology, Ningbo 315016, Zhejiang, China
First published on 17th May 2016
Abstract
A simple method, i.e. the calcination of a mixture of Co(NO3)2, PEG-PPG-PEG Pluronic® P-123, and melamine in the presence of graphene oxide (GO), has been adopted for the synthesis of Co3O4 nanoparticles encapsulated in a graphene supported carbon matrix (Co3O4@C/graphene). It shows that the co-existence of P123, melamine, and GO was crucial to obtain the small sized Co3O4 nanoparticles encapsulated in the graphene supported carbon matrix. The resulting Co3O4@C/graphene was found to be highly active for the oxygen reduction reaction and exhibited higher electrocatalytic activity and better stability than the commercial Pt/C with 20 wt% Pt loading. Their high electrocatalytic activity could be attributed to the synergistic coupling between Co3O4 and the nitrogen-doped carbon matrix/graphene, the small Co3O4 nanoparticle size, and the presence of the nitrogen doped porous carbon matrix and the carbon encapsulated structure, which make the Co3O4@C/graphene kinetically facile and highly stable during complicated electrochemical processes.
1. Introduction
Fuel cells and metal–air batteries have been considered as promising energy conversion and storage devices with great potential to alleviate the ever-increasing pressure from the energy crisis.1–4 However, their widespread use in practical applications have been greatly hampered by the sluggish oxygen reduction reaction (ORR), which is an important process in fuel cells and metal–air batteries.4–8 The traditional fuel cells and metal–air batteries use noble metal nanoparticles, such as Pt and its alloys, as the electrocatalysts for the ORR.9–13 Although these nanoparticles exhibit high catalytic activity for the ORR and are also currently being optimized,14–16 they still suffer from some severe problems, such as poor stability in electrochemical environments, low tolerance to poisoning effects, etc.17–20 These, along with their high costs and scarcity in nature, greatly hinder the large scale production and commercialization of the noble metal-loaded fuel cells or metal–air batteries. Recent efforts have therefore turned to developing noble metal-free or even metal-free catalysts.21–24Among various catalysts reported to date, transition-metal oxides (TMOs) have received a great deal of attention due to their potential uses as the electrocatalysts for the ORR.7,25–31 However, the TMOs prepared by the traditional synthetic routes usually exhibit low electrocatalytic activity due to their large particle sizes, low specific surface areas and poor electric conductivity. To improve their electrocatalytic activities, the TMOs are usually nanostructured and deposited on the highly electrical conductive carbonaceous materials.25,27,32 Since nanostructuring allows for the better accessibility of the TMOs to the ORR, while the carbonaceous materials could facilitate the electron transfer during the electrochemical reactions, the carbonaceous materials supported TMOs (TMOs/CM) are therefore expected to exhibit improved activities for the ORR. As reported by Dai et al.,25,27 the Co3O4 nanoparticles deposited on the surface of graphene or carbon nanotubes are highly efficient for the ORR. In-depth analysis has demonstrated a strong coupling between Co3O4 and graphene/CNT, which synergistically leads to the high electroactivities of the graphene/carbon nanotubes supported Co3O4 nanoparticles for the ORR. Up to now, although some work has demonstrated the great potential of using TMOs/CM as the electrocatalyst for the ORR, their fabrications usually require a relatively complicated procedure and are also not suitable for the large scale production,33–36 which would greatly limited the uses of the TMOs/CM in the practical applications. This indicates that further efforts are still needed to develop methods for the preparation of the TMOs/CM or other electrocatalysts with high efficiency for the ORR.
In this work, we developed a simple method for the synthesis of the Co3O4/CM by the calcination of a mixture of Co(NO3)2, P123, and melamine in the presence of GO. The resulting product shows a structure consisting of the Co3O4 nanoparticles encapsulated in the graphene supported carbon matrix (Co3O4@carbon/graphene). This is different from the Co3O4/graphene composites reported previously,26,27,32,37 in which the Co3O4 nanoparticles are directly deposited on the surface of the graphene nanosheets without the carbon encapsulation. The encapsulation makes the Co3O4 nanoparticles more stable during the electrochemical processes. The experimental results show that the Co3O4@C/graphene could exhibit higher electrocatalytic activity and better stability than the commercial Pt/C catalysts.
2. Experimental section
2.1 Chemicals and reagents
Flake graphite (325 meshes) was obtained from Alfa. Sodium nitrate (NaNO3, 99%), potassium permanganate (KMnO4, 99.5%), concentrated sulphuric acid (H2SO4, 95%), hydrochloric acid, hydrogen peroxide aqueous solution (H2O2, 35%), cobalt(II) nitrate hexahydrate, melamine were obtained from Guangzhou Chemical Reagent Co. Ltd. Potassium hydroxide (KOH, 85%) and methanol were obtained from Tianjin chemical reagent Co. Ltd. PEG-PPG-PEG Pluronic® P-123 (Mw = 5800) was purchased from Sigma-Aldrich. Nafion (5.0 wt%) was purchased from DuPont Company. All the chemicals were used as received without further purification. Deionized (DI) water with a resistance of ∼18 MW cm−1 was used in the reactions.2.2 Synthesis of the Co3O4@C/graphene
For a typical preparation, 2.0 mL of 0.1 g mL−1 P123, 1.0 mL of 0.3 g mL−1 melamine, and 49.3 mg of Co(NO3)2·6H2O were mixed with 8.0 mL of ∼7.5 g L−1 GO synthesized from the modified Hummers method.38–40 The obtained mixture was vigorously stirred for 2 h and then heated to 80 °C for the evaporation of the solvent. The resulting solid powder was heated to 180, 240, and 800 °C under the N2 flow and left at these temperatures for 2, 2, 1 h, respectively. After cooling, the product was washed with water and centrifuged for several times, and finally it was dried in an oven at 60 °C. For the simplicity and brevity, the fabricated catalyst was named as CoPMG, representing the use of Co(NO3)2, P123, melamine, and GO as the precursors for the synthesis of the sample.The same procedure was also used to synthesize the PMG, the CoMG, the CoPG and the CoPM in the absence of Co(NO3)2, P123, melamine or GO, respectively, while keeping other parameters constant.
2.3 Physical characterizations
An environmental scanning electron microscope (SEM) at an operation voltage of 20.0 kV was employed to detect the morphologies of the obtained samples. TEM measurements were carried out on a JEM-2100F high-resolution transmission electron microscope with an accelerating voltage of 200 kV. The chemical compositions of the samples were determined by X-ray photoelectron spectroscopy (XPS) on a PHI X-tool (Japan), using an Al K X-ray source (1486 eV). X-ray diffraction patterns (XRD) were collected using a Bruker D8 with Cu Ka radiation in a 2θ range of 20–90° (step size 0.02°, scan speed 10° min−1) and were analyzed via Bruker EVA and Bruker TopAs 4.2 software. Raman data were collected with a Renishaw in Vita Raman spectrometer with an excitation wavelength of 514.5 nm. The specific surface areas of the samples were measured by a surface area analyzer (NOVA 2000, Quantachrome) based upon the N2 adsorption/desorption isotherms recorded at the liquid-N2 temperature. The thermogravimetric (TG) analyses used to determine the relative weight percentage of Co3O4 in the CoPMG were carried out on a STA 449C under a stream of air at a heating rate of 5 °C min−1.2.4 Electrochemical measurement
5 mg of the catalysts fabricated above were dispersed in a mixture (1.0 mL) of DI water and isopropanol with a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), followed by the addition of 20 μL of Nafion (5 wt% solution in a mixture of isopropyl alcohol and water). The obtained mixture was sonicated at 100 W for 3 h to get a homogeneous ink. 2 μL of catalyst ink was then dropped onto a glassy carbon electrode (GCE) of 5 mm in diameter (0.19625 cm2 of geometric area) and dried at room temperature, which led to a catalyst loading of 0.051 mg cm−2 on the electrode. The electrode loaded with the commercial Johnson Matthey (JM) Pt/C 20 wt% (20 wt% Pt on the carbon) was also prepared using the same procedure.
1 (v/v), followed by the addition of 20 μL of Nafion (5 wt% solution in a mixture of isopropyl alcohol and water). The obtained mixture was sonicated at 100 W for 3 h to get a homogeneous ink. 2 μL of catalyst ink was then dropped onto a glassy carbon electrode (GCE) of 5 mm in diameter (0.19625 cm2 of geometric area) and dried at room temperature, which led to a catalyst loading of 0.051 mg cm−2 on the electrode. The electrode loaded with the commercial Johnson Matthey (JM) Pt/C 20 wt% (20 wt% Pt on the carbon) was also prepared using the same procedure.
Electrochemical measurements were performed using a computer-controlled potentiostat (CHI 660E, China) with a standard three-electrode cell. A platinum wire was employed as the counter-electrode and a saturated Ag/AgCl electrode as the reference electrode. All the electrochemical experiments were carried out at room temperature. The electrolytes were saturated with O2 or N2 by bubbling O2 or N2 at least 30 min before electrochemical measurements. The O2 or N2 flow was maintained over the electrolyte to ensure the O2 or N2 saturation during the recording. The polarization curves were obtained by performing a negative-direction sweep of potential at a rate of 5 mV s−1 from 0.3 V to −0.8 V (vs. Ag/AgCl) in the O2 saturated 0.10 M KOH. The working electrode was activated by potential cycling in 0.1 M KOH from 0.3 V to −0.8 V for 30 cycles at a scan rate of 10 mV s−1 before the data collection.
3. Results and discussion
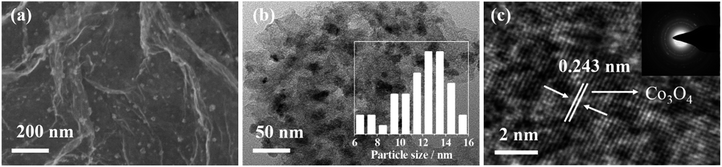
As described in the experimental section, the CoPMG was synthesized by the simple calcination of the mixture of Co(NO3)2, P123, and melamine in the presence of GO. The high temperature calcination promoted the pyrolysis of Co(NO3)2 and its reaction with oxygen containing compounds in the mixture, which would lead to the formation of the Co3O4@C/graphene due to the simultaneous carbonization of P123 and melamine and thermal reduction of the GO. Fig. 1a shows a typical SEM image of the CoPMG, in which the well-separated small particles deposited on the graphene gossamer could be clearly observed. The encapsulation of the Co3O4 nanoparticles in the carbon matrix could be demonstrated by their TEM image in Fig. 1b, which shows the embedment of the Co3O4 nanoparticles in the carbon matrix. The average size of the Co3O4 particles measured from the TEM image is ∼13 nm, as indicated by the histogram shown in inset of Fig. 1b. These Co3O4 nanoparticles are well crystallized with the lattice fringes clearly observable, as demonstrated by their high-resolution TEM image in Fig. 1c. The 0.243 nm crystal spacing corresponds to the (311) crystal plane spacing of the cubic phase Co3O4. These results are in good agreement with the electron diffraction image of the Co3O4 particles shown in the inset of Fig. 1c, in which the diffraction rings corresponding to the cubic phase Co3O4 could be clearly identified. The control experiment showed that the carbon matrix was mainly formed from the carbonization of P123. This could be demonstrated by the SEM and TEM images of the CoMG synthesized from the mixture of Co(NO3)2, melamine, and GO in the absence of P123, in which the Co3O4 nanoparticle aggregates unsurrounded by carbon could be observed (Fig. S1a and b†). This indicates that the presence of P123 in the reaction mixture could not only provide the carbon matrix for the Co3O4 nanoparticle, but also facilitate the dispersion of the Co3O4 nanoparticles on the graphene nanosheets.The XRD pattern of the CoPMG shows the (111), (220), (311), (400), (511), and (440) reflections at 2θ = 18.8, 31.1, 36.8, 44.6, 59.2, and 65.1°, respectively, characteristic of the cubic phase Co3O4 (JCPDS no. 42-1467). The peak centered at 2θ = ∼22.6° is close to the (001) reflection of the layer-by-layer stacked graphene reported previously,41,42 suggesting the presence of the graphene in the CoPMG. The broad peak profile suggests that the graphene nanosheets are not well aligned in the CoPMG. This is consistent with the fact that the CoPMG was synthesized from the calcination of the mixture of Co(NO3)2, P123, and melamine in the presence of GO, in which the GO was randomly aligned with the adsorption of the Co(NO3)2, P123, and melamine on its surface. The calcination would therefore lead to the formation of the carbon matrix with the Co3O4 nanoparticles encapsulated on the surface of the graphene nanosheets due to the carbonization of P123 and melamine and the production of the Co3O4 nanoparticle, preventing the graphene nanosheets from the layer-by-layered alignment. The absence of the diffraction peaks attributed to the other phases or impurities, indicating the high purification of the Co3O4@C/graphene. This is contrast to the XRD pattern of the CoPM synthesized from the mixture of Co(NO3)2, P123, and melamine in the absence of GO, in which a combination of the diffraction peaks from the Co3O4 and Co phases (Fig. 2) could be observed, indicating that the presence of the GO in the reaction precursor is crucial to the obtained Co3O4, since its oxygeneous groups in the GO provide enough oxygen for the formation of Co3O4. Due to the absence of graphene, the XRD pattern of the CoPM does not show the distinct reflection at 2θ = ∼22.6° (Fig. S2†). The formation of the pure Co3O4 phase promoted by the GO could further be demonstrated by the XRD patterns of the CoMG and the CoPG synthesized in the absence of P123 and melamine, respectively, where only the diffraction peaks assignable to graphene and the cubic phase Co3O4 could be identified, as shown in Fig. 2.
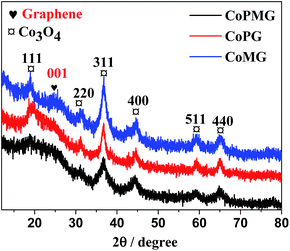 | ||
| Fig. 2 XRD patterns of the CoPMG, the CoPG, and the CoMG. In the figure, the reflection peaks attributed to Co3O4 and graphene are marked. | ||
The XPS analysis reveals the containing of Co, O, N, and C in the CoPMG (Fig. 3a). The presence of Co3O4 could be demonstrated by the two peaks locating at binding energies of 780.7 and 796.6 eV (Fig. 3b), corresponding to Co 2p3/2 and Co 2p1/2, respectively. This is further supported by its Raman spectrum shown in Fig. 4a, where four peaks at 465, 509, 602, and 668 cm−1, corresponding to Eg, F12g, F12g, and A1g modes of the crystalline Co3O4, could be observed.43 The trace amount of N in the CoPMG comes from melamine. Previous work has reported that the calcination of the carbon precursors in the presence of melamine would lead to the formation of the carbon materials with nitrogen doped through the complicated pyrolytic reactions.44–46 The absence of the peak attributed to Co–Nx in Fig. 3b indicates that Co3O4 in the CoPMG is not doped with N. The spectra deconvolution of C 1s in Fig. 3c shows the domination of the graphitic carbon, supporting the presence of the graphitic structure in the CoPMG. This could be further confirmed by its Raman spectrum in Fig. 4a, where two distinct peaks assignable to the G and D bands of the graphitic structure could be visualized. Five other peaks in the deconvoluted C 1s spectrum (Fig. 3c) at the binding energies of 284.9, 286.1, 287.7, 289.1, 291.7 eV could be attributed to C–OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C–O, C–N/C
N/C–O, C–N/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O
O, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, and the π–π* shakeup satellite peak, respectively. The presence of the peaks assigned to C
C–O, and the π–π* shakeup satellite peak, respectively. The presence of the peaks assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N further demonstrates that nitrogen has been doped into the carbon materials. The deconvoluted N 1s spectrum in Fig. 3d shows the existence of four nitrogen-containing components, corresponding to pyridinic (398.2 eV), pyrrolic (400.0 eV), graphitic (401.5 eV), and oxidized (403.7 eV) type N-functionalities, respectively. This is in good agreement with nitrogen doped carbon materials reported previously.46–48 However, due to the structure complexity of the CoPMG, the doping of nitrogen in the carbon matrix of the Co3O4 nanoparticles or the graphitic structure of graphene could not well be resolved. We assume that both the carbon matrix and the graphitic structure of graphene are doped with nitrogen since the CoPMG was synthesized by the calcination of the well mixed mixture of Co(NO3)2, P123, melamine, and GO. The control experiment has shown that the presence of melamine is important to obtain the Co3O4 nanoparticles with relatively small sizes. Otherwise, the Co3O4 nanoparticles with much larger sizes would be fabricated, as demonstrated by the SEM and TEM images of CoPG shown in Fig. S1c and d.† This is possible since the doped nitrogen obtained from the pyrolytic reactions between melamine and P123/GO could act as favorable nucleation and anchoring sites for the Co3O4 nanoparticles due to the strong interaction between nitrogen and Co2+, preventing them from growing into larger particles during the calcination. The appearance of the XRD peaks of the CoPMG at the same positions with those of the CoPG, as shown in Fig. 2, further suggests that Co3O4 in the CoPMG is not doped with N. The TGA analysis shows that the weight percentage of Co3O4 in the CoPMG is ∼36% (Fig. S3†).
N and C–N further demonstrates that nitrogen has been doped into the carbon materials. The deconvoluted N 1s spectrum in Fig. 3d shows the existence of four nitrogen-containing components, corresponding to pyridinic (398.2 eV), pyrrolic (400.0 eV), graphitic (401.5 eV), and oxidized (403.7 eV) type N-functionalities, respectively. This is in good agreement with nitrogen doped carbon materials reported previously.46–48 However, due to the structure complexity of the CoPMG, the doping of nitrogen in the carbon matrix of the Co3O4 nanoparticles or the graphitic structure of graphene could not well be resolved. We assume that both the carbon matrix and the graphitic structure of graphene are doped with nitrogen since the CoPMG was synthesized by the calcination of the well mixed mixture of Co(NO3)2, P123, melamine, and GO. The control experiment has shown that the presence of melamine is important to obtain the Co3O4 nanoparticles with relatively small sizes. Otherwise, the Co3O4 nanoparticles with much larger sizes would be fabricated, as demonstrated by the SEM and TEM images of CoPG shown in Fig. S1c and d.† This is possible since the doped nitrogen obtained from the pyrolytic reactions between melamine and P123/GO could act as favorable nucleation and anchoring sites for the Co3O4 nanoparticles due to the strong interaction between nitrogen and Co2+, preventing them from growing into larger particles during the calcination. The appearance of the XRD peaks of the CoPMG at the same positions with those of the CoPG, as shown in Fig. 2, further suggests that Co3O4 in the CoPMG is not doped with N. The TGA analysis shows that the weight percentage of Co3O4 in the CoPMG is ∼36% (Fig. S3†).
The N2 adsorption–desorption isotherms of the CoPMG exhibits a type II hysteresis with a rapid uptake in the P/P0 region of 0 to 0.04 in Fig. 4b, indicating the presence of pores in the CoPMG. Based upon the adsorption–desorption isotherm of the CoPMG, it is extracted that the Brunauer–Emmett–Teller (BET) specific surface area of the CoPMG is 410.6 m2 g−1, which is significantly larger than other graphene supported TMO nanoparticles reported previously.49,50 The high surface area and porous structure make the CoPMG more fascinating as the electrocatalyst for the ORR, since the high specific surface area would make more active sites accessible to the ORR, while the porous structure allows a ready diffusion of electrolytes.
To demonstrate that the CoPMG fabricated above are electrochemically active for the ORR, its CV curves in the N2 and O2 saturated 0.1 M KOH solutions were first measured. Fig. 5a shows that the CV curve of the CoPMG obtained from the N2-saturated solution shows featureless cathodic voltammetric currents in the potential range covered in this work, while its corresponding CV curve in the O2 saturated solution exhibits a well-defined cathodic peak corresponding to the reduction of oxygen (the anodic peaks in the CV curves of the CoPMG could be attributed to the redox reactions associated with the Co3O4 nanoparticles26,51). This clearly demonstrates the electrocatalytic activity of the CoPMG for the ORR. In addition, the electrocatalytic activity of the CoPMG for the ORR could further be demonstrated by its linear sweep voltammetric (LSV) curves measured by the rotating disk electrode in Fig. 5b, where the voltammetric current ascribable to the reduction of oxygen could be clearly identified. Fig. 5b shows that although the onset potential for the ORR by the CoPMG is comparable to that of the commercial Pt/C 20 wt%, its half-wave potential appears at a position positive to that of the commercial Pt/C 20 wt%, indicating a higher electrocatalytic activity of the CoPMG than that of the commercial Pt/C 20 wt%. The higher electrocatalytic activity of the CoPMG for the ORR could further be demonstrated by its higher limiting current density than that of the commercial Pt/C 20 wt%, as shown in Fig. 5b. These results clearly demonstrate that the CoPMG could be used as an efficient catalyst for the ORR.
 | ||
| Fig. 5 (a) CV curves of the CoPMG, the CoPM, the CoPG, the CoMG, and the PMG in the N2 and O2-saturated 0.1 M KOH solution at the scan of 10 mV s−1 (b) LSV curves of the CoPMG, the CoPM, the CoPG, the CoMG, the PMG, and the Pt/C 20 wt% for the ORR in the O2-saturated 0.1 M KOH solution at the scan of 10 mV s−1 and the rotation rate of 1600 rpm. (c) LSV curves at various rotation rates for the ORR by the CoPMG in the O2-saturated 0.1 M KOH solution. (d) K–L plots of the ORR by the CoPMG obtained based on the data extracted from (c). The dots are the experimental results and the straight lines are their corresponding fittings using eqn (1). (e) Numbers of electron transfer and (f) calculated kinetic current densities involved in the ORR by the CoPMG, the CoPM, the CoPG, the CoMG, the PMG, and the Pt/C 20 wt% for in the O2-saturated 0.1 M KOH. In all the cases, the mass loading of the catalysts are ∼0.051 mg cm−2. | ||
To understand the origin of the high electrocatalytic activity of the CoPMG, the electrocatalytic activities of the CoPM, the CoPG, and CoMG for the ORR were also investigated. Fig. 5b shows that although the CoPM, the CoPG, and CoMG are all electrochemically active for the ORR, their activities are much lower than that of the CoPMG, which could be clearly demonstrated by their more negative onset and half-wave potentials and much lower limiting current densities. This strongly suggest that the calcination of Co(NO3)2 in the co-existence of P123, melamine, and GO, which leads to the formation of the CoPMG with the relatively small size of the Co3O4 nanoparticles and nitrogen doped structure, is crucial to obtain the electrocatalyst with higher activity. The exclusion of any single reaction component, such as P123, melamine or GO, from the mixture, which produces the graphene supported Co3O4 nanoparticle aggregates unencapsulated by the carbon matrix (CoMG), the Co3O4@C/graphene with the larger Co3O4 nanoparticles and without nitrogen doped structure (CoPG), or the Co nanoparticles encapsulated in the carbon matrix without graphene (CoPM), as demonstrated above, would lead to the formation of the catalysts with relatively low electrocatalytic activities. This might be due to the reasons that the aggregation and the large particle sizes reduce the accessibility of Co3O4 to the ORR, while the Co nanoparticles are not favored for the high efficient ORR.
Prior studies have reported that Co–Nx could be a possible active site for the ORR,52 which might contribute to the high electrocatalytic activity of the CoPMG observed above. However, the deconvoluted XPS spectra of Co 2p and N 1s in Fig. 3c and d show the absence of Co–Nx in the CoPMG, which clearly rule out the possibility of the contribution of Co–Nx to the high electrocatalytic activity of the CoPMG. This could also be demonstrated by the XPS spectra of the PMG synthesized from the etching of the CoPMG with 1.0 M H2SO4 in Fig. 3a (the TEM image of the PMG is shown in Fig. S4†), where no peaks assignable to Co could be observed (the Co–Nx is relative stable in the H2SO4 solution since it is electrocatalytically active for the ORR in the acid solution, as reported previously52).
Since the PMG was synthesized from the etching of the CoPMG with 1.0 M H2SO4, it possesses a nitrogen doped structure, as shown in Fig. 3a. Fig. 5a and b show that the PMG is also electrocatalytically active for the ORR, since the nitrogen doping would create net positive charges on the adjacent carbon atoms, facilitating the oxygen adsorption and the subsequent ORR, as reported previously.47,53 Worthnoting is that although the PMG is electrocatalytically active for the ORR, its activity is much lower than that of the CoPMG, as demonstrated by its less positive onset potential and lower limiting current density for the ORR shown in Fig. 5b. This indicates that the Co3O4 nanoparticles play an important role in the high electrocatalytic activity of the CoPMG. Previous work has demonstrated that the carbon shell synthesized from the calcination of organic compounds or polymers usually exhibit amorphous porous morphology.54,55 The porous feature would make the Co3O4 nanoparticles encapsulated in the carbon matrix well accessible to electrolyte, participating in the ORR. This indicates that despite the encapsulation in the carbon matrix the Co3O4 nanoparticles are still available for the ORR. Up to now, although Co3O4 has been reported to be electrochemically active for the ORR, its activity is relatively low due to its low electric conductivity.7,56 These results make us believe that the electrochemical activity of the CoPMG arises from both the PMG and the Co3O4 nanoparticles, while the synergistic coupling between Co3O4 and PMG might impose a big contribution on the high electrocatalytic activity of the CoPMG. As reported previously,25,27 the deposition of TMO onto the nitrogen doped graphene would lead to a strong coupling between TMO and nitrogen doped graphene, which could increase the electrocatalytic activity of TMO.
To gain further insight into the ORR catalyzed by the CoPMG, its LSVs in the O2-saturated KOH solutions under different electrode rotating rates were measured. For comparison, the LSVs of the CoPM, the CoPG, the CoMG, the PMG, and the Pt/C 20 wt% in the O2-saturated 0.1 M KOH solutions under various electrode rotating rates were also measured. Fig. 5c and S3† show an enhancement of the ORR current density with increase of the rotating rate, due to the improved electrolyte diffusion. The ORR performance in the diffusion and kinetic limited regions was then analyzed using the Koutecky–Levich (K–L) equations shown below, and the slopes of the best linear fits for the K–L plots shown in Fig. 5d and S3† are used for the calculation of the number of electron transferred during the ORR.
 | (1) |
| B = 0.62nFC0D2/3υ−1/6; jk = nFkC0 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 C mol−1), C0 is the O2 saturated concentration in 0.1 M KOH (C0 = 1.2 × 10−6 mol cm−3), υ is the kinematic viscosity of the electrolyte (0.01 cm2 s−1), D is the O2 diffusion coefficient of the electrolyte (D = 1.9 × 10−5 cm2 s−1), and k is the electro-transfer rate constant. Fig. 5e and f shows the electron transfer numbers and calculated kinetic current densities involved in the ORR by the CoPMG, the CoPM, the CoPG, the CoMG, the PMG, and the commercial JM Pt/C wt 20% based on eqn (1) and (2) (the LSV curves at various rotation rates and the K–L plots of the CoPM, the CoPG, the CoMG, the PMG, and the Pt/C 20 wt% for the ORR are given in the ESI, Fig. S5†).
486 C mol−1), C0 is the O2 saturated concentration in 0.1 M KOH (C0 = 1.2 × 10−6 mol cm−3), υ is the kinematic viscosity of the electrolyte (0.01 cm2 s−1), D is the O2 diffusion coefficient of the electrolyte (D = 1.9 × 10−5 cm2 s−1), and k is the electro-transfer rate constant. Fig. 5e and f shows the electron transfer numbers and calculated kinetic current densities involved in the ORR by the CoPMG, the CoPM, the CoPG, the CoMG, the PMG, and the commercial JM Pt/C wt 20% based on eqn (1) and (2) (the LSV curves at various rotation rates and the K–L plots of the CoPM, the CoPG, the CoMG, the PMG, and the Pt/C 20 wt% for the ORR are given in the ESI, Fig. S5†).
The electrocatalytic oxygen reduction in an alkaline solution occurs either via in a two-electron pathway involving the formation of H2O2 as an intermediate or via a four-electron pathway in which O2 is directly reduced to OH−.57,58 Generally, the four-electron pathway is preferred because it provides a faster rate for the ORR. Fig. 5e shows that the number of electron transfer involved in the ORR by the CoPMG is close to 4 over the potential range, indicating that the ORR catalyzed by the CoPMG mainly proceeds via a four-electron process. The relatively lower electron transfer numbers involved in the ORR by the CoPM, the CoPG, the CoMG, and the PMG suggests that the two-electron pathway is playing an increasing role in the reduction of oxygen. The high electron transfer number, in combination with the relatively higher kinetic current density of the ORR by the CoPMG in comparison to those by the CoPM, the CoPG, and the PMG further suggests that the CoPMG is a more efficient electrocatalyst for the ORR. The most interesting is that although the electron transfer number of the ORR by the Pt/C 20 wt% is also close to 4 over the measured potential range, its kinetic current density is much lower than that of the CoPMG. This well explains why the CoPMG could exhibit high electrocatalytic activity than the Pt/C 20 wt%, as shown in Fig. 5b. All these results clearly demonstrate that the CoPMG could be used as an efficient catalyst for the ORR with great potential to replace the current noble metal based catalysts.
Indeed, the potential use of the CoPMG as the catalyst for the ORR could further demonstrated by its high stability and good immunity toward the methanol crossover. Fig. 6a shows that the CoPMG could remain highly efficient for the ORR with only a loss of <10% of its original activity after 10 hs of the ORR. The introduction of methanol shows no influence on its catalytic activity. This is unlike the Pt/C 20 wt%, in which a loss of over 17% of its original activity could be observed after 10 hs of the ORR and the introduction of methanol shows even greater losses of its activity, due to the detachment of the Pt nanoparticles from the carbon substrate or their aggregation during the electrochemical processes and the blockage of active sites on the Pt nanoparticles by the methanol oxidation products.59–61 The high stability of the CoPMG might be attributed to the fact that the Co3O4 nanoparticles of the CoPMG are encapsulated in the carbon matrix, which could prevent them from the dissociation and/or detachment during the complicated electrocatalytic processes. This is unlike the CoMG in which the Co3O4 nanoparticle aggregates unincapsulated by the carbon matrix are directly deposited on the surface of the graphene nanosheets. The ORR might lead to the dissociation and/or detachment of the Co3O4 nanoparticle aggregates from the graphene nanosheets. As shown in Fig. 6a, a relatively higher loss in the activity of the CoMG (>30%) could be observed after 10 h of the ORR.
Based on the structural information and detailed analysis shown above, the following aspects could be attributed to the possible reasons leading to the high electrocatalytic activity for the CoPMG: (1) small Co3O4 nanoparticles and large specific surface area of the CoPMG, which make more active materials accessible to the ORR; (2) nitrogen doped porous carbon matrix (3) synergistic coupling between Co3O4 and PMG, which make them more active for the ORR; (4) carbon encapsulated structure, which prevents the Co3O4 nanoparticles from the dissociation and/or detachment during the complicated electrocatalytic processes.
4. Conclusions
In summary, the Co3O4@C/graphene has synthesized by a simple calcination of the mixture of Co(NO3)2, P123, melamine, and GO. It shows that the co-existence of P123, melamine, and GO in the calcination of Co(NO3)2 is crucial to obtain the Co3O4@C/graphene with the relatively higher electrocatalytic activity for the ORR. The exclusion of any reaction component would lead to the formation of the catalysts with greatly reduced activities for the ORR. Analysis has demonstrated that the synergistic coupling between Co3O4 and nitrogen-doped carbon/graphene, the smaller Co3O4 nanoparticle sizes, and the presence of the nitrogen doped porous carbon matrix and the carbon encapsulation structure play important roles in the high electrochemical performance of the Co3O4@C/graphene. Indeed, the Co3O4@C/graphene can even exhibit higher electrocatalytic activity and better stability than the commercial Pt/C catalysts, which makes the Co3O4@C/graphene particularly attractive as the electrocatalysts for the ORR. The present work is therefore of great interest since it provides a simple method for the synthesis of the catalyst with the significantly higher ORR activity.Acknowledgements
This work was financially supported by the “Outstanding Talent and Team Plans Program” of South China University of Technology, the Chinese National Natural Science Foundation (No. 11474101, and U1532139), the Zhejiang Provincial Natural Science Foundation (No. LY14B030001), the Zhejiang Provincial Public Welfare Technology Application Research Project (2015C31151), and the Ningbo Natural Science Foundation (No. 2014A610035), State Key Laboratory of Pollution Control and Resource Reuse (No. PCRRF14006) and State Key Lab of Subtropical Building Science, South China University of Technology (2014KB13).Notes and references
- H. Zhang and P. K. Shen, Chem. Rev., 2012, 112, 2780–2832 CrossRef CAS PubMed.
- R. Devanathan, Energy Environ. Sci., 2008, 1, 101–119 CAS.
- J.-S. Lee, S. T. Kim, R. Cao, N.-S. Choi, M. Liu, K. T. Lee and J. Cho, Adv. Energy Mater., 2011, 1, 34–50 CrossRef CAS.
- F. Cheng and J. Chen, Chem. Soc. Rev., 2012, 41, 2172–2192 RSC.
- M. Lefevre, E. Proietti, F. Jaouen and J. P. Dodelet, Science, 2009, 324, 71–74 CrossRef CAS PubMed.
- J.-I. J. H. Y. Jeong, J.-S. Lee, M. G. Kim and J. Cho, Angew. Chem., 2014, 126, 4670–4674 CrossRef.
- J. Xu, P. Gao and T. S. Zhao, Energy Environ. Sci., 2012, 5, 5333–5339 CAS.
- Q. Liu, J. Jin and J. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 5002–5008 CAS.
- J. W. Hong, S. W. Kang, B.-S. Choi, D. Kim, S. B. Lee and S. W. Han, ACS Nano, 2012, 6, 2410–2419 CrossRef CAS PubMed.
- D. Wang, H. L. Xin, Y. Yu, H. Wang, E. Rus, D. A. Muller and H. D. Abruna, J. Am. Chem. Soc., 2010, 132, 17664–17666 CrossRef CAS PubMed.
- S. Guo and S. Sun, J. Am. Chem. Soc., 2012, 134, 2492–2495 CrossRef CAS PubMed.
- X. Wang, M. I. Vara, M. Luo, H. Huang, A. Ruditskiy, J. Park, S.-X. Bao, J. Liu, J. Y. Howe, M. Chi, Z.-X. Xie and Y. Xia, J. Am. Chem. Soc., 2015, 137, 15036–15042 CrossRef CAS PubMed.
- L. Zhang, R. Iyyamperumal, D. F. Yancey, R. M. Crooks and G. Henkelman, ACS Nano, 2013, 7, 9168–9172 CrossRef CAS PubMed.
- S. Guo, D. Li, H. Zhu, S. Zhang, N. M. Markovic, V. R. Stamenkovic and S. Sun, Angew. Chem., Int. Ed., 2013, 52, 3465–3468 CrossRef CAS PubMed.
- Q. Jiang, L. Jiang, S. Wang, J. Qi and G. Sun, Catal. Commun., 2010, 12, 67–70 CrossRef CAS.
- S. S. Aravind and S. Ramaprabhu, ACS Appl. Mater. Interfaces, 2012, 4, 3805–3810 CAS.
- S. M. Alia, K. O. Jensen, B. S. Pivovar and Y. Yan, ACS Catal., 2012, 2, 858–863 CrossRef CAS.
- C. Wang, H. Daimon, T. Onodera, T. Koda and S. Sun, Angew. Chem., Int. Ed., 2008, 47, 3588–3591 CrossRef CAS PubMed.
- V. R. Stamenkovic, B. Fowler, B. S. Mun, G. Wang, P. N. Ross, C. A. Lucas and N. M. Marković, Science, 2007, 315, 493–497 CrossRef CAS PubMed.
- L. Xiao, L. Zhuang, Y. Liu, J. Lu and H. D. Abruña, J. Am. Chem. Soc., 2009, 131, 602–608 CrossRef CAS PubMed.
- W. Yuan, J. Li, L. Wang, P. Chen, A. Xie and Y. Shen, ACS Appl. Mater. Interfaces, 2014, 6, 21978–21985 CAS.
- C. H. Choi, M. W. Chung, H. C. Kwon, S. H. Park and S. I. Woo, J. Mater. Chem. A, 2013, 1, 3694–3699 CAS.
- Z. Jiang, Z.-j. Jiang, X. Tian and W. Chen, J. Mater. Chem. A, 2014, 2, 441–450 CAS.
- L. Dai, Y. Xue, L. Qu, H.-J. Choi and J.-B. Baek, Chem. Rev., 2015, 115, 4823–4892 CrossRef CAS PubMed.
- Y. Liang, H. Wang, J. Zhou, Y. Li, J. Wang, T. Regier and H. Dai, J. Am. Chem. Soc., 2012, 134, 3517–3523 CrossRef CAS PubMed.
- Y. Liang, H. Wang, P. Diao, W. Chang, G. Hong, Y. Li, M. Gong, L. Xie, J. Zhou, J. Wang, T. Z. Regier, F. Wei and H. Dai, J. Am. Chem. Soc., 2012, 134, 15849–15857 CrossRef CAS PubMed.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Nat. Mater., 2011, 10, 780–786 CrossRef CAS PubMed.
- G. Wu and P. Zelenay, Acc. Chem. Res., 2013, 46, 1878–1889 CrossRef CAS PubMed.
- S.-A. Park, H. Lim and Y.-T. Kim, ACS Catal., 2015, 5, 3995–4002 CrossRef CAS.
- K. Selvakumar, S. M. S. Kumar, R. Thangamuthu, K. Ganesan, P. Murugan, P. Rajput, S. N. Jha and D. Bhattacharyya, J. Phys. Chem. C, 2015, 119, 6604–6618 CAS.
- W. Xiao, D. Wang and X. W. Lou, J. Phys. Chem. C, 2010, 114, 1694–1700 CAS.
- T. Odedairo, X. Yan, J. Ma, Y. Jiao, X. Yao, A. Du and Z. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 21373–21380 CAS.
- M. Sun, Y. Dong, G. Zhang, J. Qu and J. Li, J. Mater. Chem. A, 2014, 2, 13635–13640 CAS.
- S. Ren, S. Ma, Y. Yang, Q. Mao and C. Hao, Electrochim. Acta, 2015, 178, 179–189 CrossRef CAS.
- W. Huang, H. Zhong, D. Li, P. Tang and Y. Feng, Electrochim. Acta, 2015, 173, 575–580 CrossRef CAS.
- W. J. Basirun, M. Sookhakian, S. Baradaran, Z. Endut, M. R. Mahmoudian, M. Ebadi, R. Yousefi, H. Ghadimi and S. Ahmed, Sci. Rep., 2015, 5, 9108 CrossRef CAS PubMed.
- K. Wang, R. Wang, H. Li, H. Wang, X. Mao, V. Linkov and S. Ji, Int. J. Hydrogen Energy, 2015, 40, 3875–3882 CrossRef CAS.
- Y. Jiang, Z.-J. Jiang, S. Cheng and M. Liu, Electrochim. Acta, 2014, 146, 437–446 CrossRef CAS.
- Z. Jiang, B. Pei and A. Manthiram, J. Mater. Chem. A, 2013, 1, 7775–7781 CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- Z. Jiang, X. Zhao, Y. Fu and A. Manthiram, J. Mater. Chem., 2012, 22, 24862–24869 RSC.
- Y. Tang, F. Huang, W. Zhao, Z. Liu and D. Wan, J. Mater. Chem., 2012, 22, 11257–11260 RSC.
- X. C. Dong, H. Xu, X. W. Wang, Y. X. Huang, M. B. Chan-Park, H. Zhang, L. H. Wang, W. Huang and P. Chen, ACS Nano, 2012, 6, 3206–3213 CrossRef CAS PubMed.
- Z. Jiang, Z.-J. Jiang, X. Tian and L. Luo, Electrochim. Acta, 2014, 146, 455–463 CrossRef CAS.
- Z.-H. Sheng, L. Shao, J.-J. Chen, W.-J. Bao, F.-B. Wang and X.-H. Xia, ACS Nano, 2011, 5, 4350–4358 CrossRef CAS PubMed.
- Z. Lin, M. K. Song, Y. Ding, Y. Liu, M. Liu and C. P. Wong, Phys. Chem. Chem. Phys., 2012, 14, 3381–3387 RSC.
- L. Qu, Y. Liu, J.-B. Baek and L. Dai, ACS Nano, 2010, 4, 1321–1326 CrossRef CAS PubMed.
- Z. Lin, G. Waller, Y. Liu, M. Liu and C.-P. Wong, Adv. Energy Mater., 2012, 2, 884–888 CrossRef CAS.
- J. Zhang, P. Li, Z. Wang, J. Qiao, D. Rooney, W. Sun and K. Sun, J. Mater. Chem. A, 2015, 3, 1504–1510 CAS.
- A. K. Rai, J. Gim, L. T. Anh and J. Kim, Electrochim. Acta, 2013, 100, 63–71 CrossRef CAS.
- I. G. Casella and M. Gatta, J. Electroanal. Chem., 2002, 534, 31–38 CrossRef CAS.
- C. W. B. Bezerra, L. Zhang, K. Lee, H. Liu, A. L. B. Marques, E. P. Marques, H. Wang and J. Zhang, Electrochim. Acta, 2008, 53, 4937–4951 CrossRef CAS.
- S. Wang, E. Iyyamperumal, A. Roy, Y. Xue, D. Yu and L. Dai, Angew. Chem., Int. Ed., 2011, 50, 11756–11760 CrossRef CAS PubMed.
- W. Song, Z. Chen, C. Yang, Z. Yang, J. Tai, Y. Nan and H. Lu, J. Mater. Chem. A, 2015, 3, 1049–1057 CAS.
- G. Nam, J. Park, M. Choi, P. Oh, S. Park, M. G. Kim, N. Park, J. Cho and J.-S. Lee, ACS Nano, 2015, 9, 6493–6501 CrossRef CAS PubMed.
- S. K. Singh, V. M. Dhavale and S. Kurungot, ACS Appl. Mater. Interfaces, 2015, 7, 442–451 CAS.
- J. D. Wiggins-Camacho and K. J. Stevenson, J. Phys. Chem. C, 2011, 115, 20002–20010 CAS.
- L. Zhang and Z. Xia, J. Phys. Chem. C, 2011, 115, 11170–11176 CAS.
- N. Wakabayashi, M. Takeichi, H. Uchida and M. Watanabe, J. Phys. Chem. B, 2005, 109, 5836–5841 CrossRef CAS PubMed.
- O. A. Baturina, S. R. Aubuchon and K. J. Wynne, Chem. Mater., 2006, 18, 1498–1504 CrossRef CAS.
- Y. Zhang, Q. Huang, Z. Zou, J. Yang, W. Vogel and H. Yang, J. Phys. Chem. C, 2010, 114, 6860–6868 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images of the CoMG and the CoPG, and TEM images of the CoMG and the CoPG; XRD patterns of the CoPM; TGA curve of the CoPMG; TEM images of the PMG synthesized through the acid etching of the CoPMG at high and low magnification; LSV curves at various different rotation rates for the ORR and corresponding K–L plots for the CoPM, the CoPG, the CoMG, the PMG, the Pt/C 20 wt% in the O2-saturated 0.1 M KOH solution. See DOI: 10.1039/c6ra09528c |
| This journal is © The Royal Society of Chemistry 2016 |