Novel phosphorus-containing halogen-free ionic liquids: effect of sulfonate anion size on physical properties, biocompatibility, and flame retardancy†
Abstract
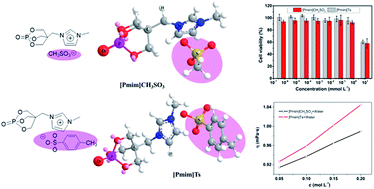
Two novel phosphorus-containing ionic liquids with different size sulfonate anions ([Pmim]CH3SO3, [Pmim]Ts) were synthesized and their physicochemical properties including thermal stability, solubility, viscosity, and melting points were comparably investigated. [Pmim]Ts, with a tosylate anion, shows a lower melting point, higher viscosity, and higher thermal stability due to the increasing anion size and uniform electron delocalization. The cytotoxicity of [Pmim]CH3SO3 and [Pmim]Ts against HeLa cells was also evaluated in vitro by MTT assay. It was found that [Pmim]CH3SO3 and [Pmim]Ts show lower toxicity than the commercial ILs with sulfate anions. Meanwhile, [Pmim]CH3SO3 and [Pmim]Ts were used as flame retardants for PA6, and it was found that they present different flame retardant effects. The limiting oxygen index (LOI) values of PA6/[Pmim]CH3SO3 and PA6/[Pmim]Ts are 26.2, and 24.4%, respectively, when their addition is 30 wt%. In the cone calorimeter test, the total heat released (THR) values of PA6 are decreased with the addition of [Pmim]CH3SO3 and [Pmim]Ts, and the burning residues from barren to abundant. Thermogravimetric analysis and thermal degradation kinetics were further investigated and it was found that both [Pmim]CH3SO3 and [Pmim]Ts can accelerate the decomposition of PA6. Because of the acidity difference of CH3SO3 and the Ts anion, [Pmim]CH3SO3, whose anion has strong acidity, shows high catalysis decomposition ability.

 Please wait while we load your content...
Please wait while we load your content...