Effect of lanthanum doping on modulating the thermochromic properties of VO2 thin films
Ning Wanga,
Nigel Tan Chew Shuna,
Martial Duchampb,
Rafal E. Dunin-Borkowskib,
Zhong Lic and
Yi Long*a
aSchool of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798, Singapore. E-mail: longyi@ntu.edu.sg
bErnst Ruska-Centre for Microscopy and Spectroscopy with Electrons (ER-C) and Peter Grünberg Institute (PGI), Forschungszentrum Jülich, 52428, Germany
cSchool of Mechanical and Aerospace Engineering, Nanyang Technological University, Nanyang Avenue, 639798 Singapore
First published on 9th May 2016
Abstract
Lanthanum (La) doped vanadium dioxide (VO2) thin films were fabricated through a facile sol–gel technique. The La doping was found to be effective for reducing the phase transition temperature (τc) of VO2 below the 4 at% doping level, with a reducing rate of −1.1 °C per at%. In addition, below the 3 at% La doping level, the band gap (Eg) decreased steadily with the increasing addition of La dopants. 5 at% La doping can enhance both the integrated visible transmission (Tlum) and the solar modulating ability (ΔTsol) simultaneously. The combination of Tlum = 50.1% and ΔTsol = 10.3% represents the best values for VO2 continuous thin films.
Introduction
Vanadium dioxide (VO2) is an interesting phase transition material with a critical temperature (τc) of around 68 °C, accompanied by a reversible metal to insulator transition (MIT).1,2 With regards to pure VO2, the insulating phase (τc < 68 °C) with a monoclinic P21/c crystal system shows a strong transmission in the infrared (IR)/near infrared (NIR) range, while the metallic counterpart (τc > 68 °C) with a tetragonal P42/mnm crystal system exhibits depressed transmission across the IR/NIR range.3,4 The thermal modulating abilities in the IR/NIR range and the electrical conductivity contrast across τc make VO2 a promising candidate for thermochromic smart windows and various smart sensor applications.5–8 Especially for smart windows, the IR/NIR light can be transmitted (τ < τc) or absorbed (τ > τc) automatically according to the outdoor temperature, maintaining a comfortable indoor environment, which meets today’s energy-saving requirements.9However, the high τc, low visible transmission (Tlum), and low solar modulating abilities (ΔTsol) of pure VO2 are three main drawbacks to be overcome.9,10 Moreover, there is always a trade-off between enhancing both Tlum and ΔTsol simultaneously.11 With respect to τc, extra strain energy12 and doping with metal cations with large ionic radii/large valence states13–15 have proved to be effective in reducing τc. As for Tlum, the microroughness,16 widening band gap (Eg),17 biomimetic nanostructure,11 nanogrid7 and porous morphology18–21 have been reported to be able to increase Tlum. Lastly, ΔTsol is always believed to be correlated to the solid contents of VO2 (ref. 22) and/or surface plasmon resonance (SPR)10,23 under some special surface/interface conditions.
Rare earth (RE) elements are characterized by their large ionic radii and abundant valence electrons, which always contribute to the enhancement of physical properties as dopants.24–26 The europium cation (Eu3+) has been found to be effective for reducing the τc of VO2 with a rate of −6.5 °C per at%, and a moderate combination of Tlum = 54% and ΔTsol = 6.7% was achieved.13 The terbium cation (Tb3+) has been reported to be helpful for increasing Tlum from ∼45% to ∼79%, but only a relatively low τc reducing rate (−1.5 °C per at%) could be obtained.27 As predicted by Chao Sun et al. using density functional theory (DFT) calculations,28 doping with the lanthanum cation (La3+) could cause the V–V distance to change and a great V–V dimer distortion, which may result in a decrease in τc. In this paper, La3+ cations were first experimentally doped into the VO2 lattice at different doping levels from 1 to 5 at%. It was found that the τc of the MIT could be reduced with a rate of −1.1 °C per at%, and a combination of Tlum = 50% and ΔTsol = 10.3% could be achieved at the 4 at% doping level.
Experimental section
All of the chemicals – V2O5 (99.6%, Alfa Aesar), La2O3 (99.9%, Alfa Aesar) and H2O2 (30 wt%, Sigma-Aldrich) – were used as received without any further purification.Precursor preparation
182 mg of V2O5 and weighed La2O3 powder (0/3.26/6.52/9.78/13.04/16.30 mg) were added into 5 mL of hot H2O2 (30 wt%, 90 °C) solution under vigorous stirring. After a violent evaporation and cooling down to room temperature, another 15 mL of H2O2 (30 wt%) was added into the suspension, and a clear brown color precursor solution was attained after stirring for 4 minutes. Then the precursor was immediately moved to do the dip coating. It should be noted that the reaction should be done in a 300 mL beaker due to the strong heat release during the reaction between V2O5 and hot H2O2.VO2 thin film fabrication
A fused silica substrate with dimensions of 15 × 15 × 0.5 mm3 was dipped into the precursor solution at a rate of 50 mm min−1. After immersing for 20 seconds, the substrate was lifted up vertically at a withdrawing rate of 200 mm min−1. After drying in air, a brownish thin film with a thickness of ∼60 nm per side was on the substrate. Then the thin film was moved into a tube furnace and annealed at 550 °C for 2 h in an Ar flow (200 mL min−1).Characterization
The phase of the products was characterized with a Shimadzu XRD-6000 X-ray diffractometer (Cu-Kα, λ = 0.15406 nm) using a voltage of 40 kV and a current of 30 mA at an X-ray grazing angle of 1.0°. Their morphology and La doping level were determined using a field emission scanning electron microscope (FESEM, JSM-7600F, JEOL, Japan) with an INCA EDX detector at an accelerating voltage of 5 kV and 20 kV, respectively. For conventional transmission electron microscopy (TEM) studies, i.e. using selected area electron diffraction (SAED) and bright field (BF) imaging, a JEOL 2010 (JEOL Company, Japan) microscope was used at an accelerating voltage of 200 kV. Transmittance spectra in the range of 250–2500 nm were measured using an UV-vis-NIR spectrophotometer (Cary 5000, Agilent Ltd, USA) equipped with a Linkam PE120 system Peltier heating & cooling stage. The integrated visible transmittance (Tlum 380–780 nm) and solar/IR transmittance (Tsol 280–2500 nm; TIR 780–2500 nm) were calculated based on the recorded % T spectra using the expression:
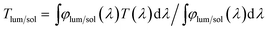 | (1) |
| % T(τ) = A2 + (A1 − A2)/[1 + exp(τ − τc)/B] | (2) |
Results and discussion
Synthesis of the La doped VO2 thin films
Fig. 1a shows the XRD patterns of the pristine and La doped VO2 thin films. The bump around 20° (2θ) in the XRD patterns should be ascribed to the diffraction of the amorphous fused silica substrates. All of the thin films exhibit the characteristic (011) XRD peak of VO2 (monoclinic, P21/c, JCPDS #82-661) around 28° (2θ), and no other impurity phase can be found, indicating the successful synthesis of the VO2 phase. Compared with the pristine VO2, the (011) peak position in the 5 at% La doped sample is left-shifted by 0.2° from 28.0° to 27.8°, which indicates the lattice expansion arising from the replacement of V by La with a relatively larger ionic radius (1.032 Å vs. 0.58 Å) in accordance with the DFT calculations.28 The La doping levels were further confirmed by EDX analysis with a 10% deviation. As depicted in Fig. 1b, the EDX results agree well with the expected doping levels. | ||
| Fig. 1 (a) XRD patterns of the pristine and La doped (1–5 at%) VO2 thin films. (b) La doping levels determined by EDX characterization with a 10% deviation. | ||
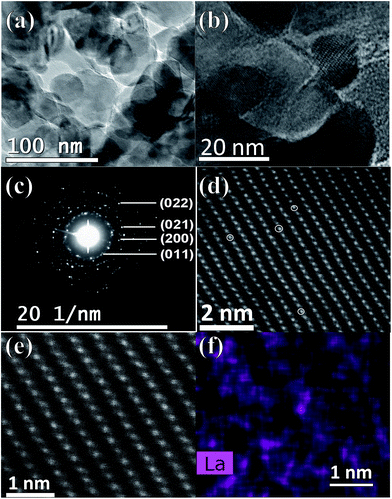
Fig. 2 shows the morphology evolution of the pristine and La doped VO2 thin films tested using FESEM. All of the samples show a nanograin packed morphology, and the average grain size for the 0–5 at% La doped samples is 61, 72, 84, 79, 62 and 73 nm, respectively, which shows that the La doping gives rise to a slight increase in the grain size. The roughness and thickness of the thin films have been detected using AFM under tapping mode, and are tabulated in Table 1. The thickness of the La-doped samples shows a deviation by ∼±14 nm compared to the pristine VO2 thin film, while the roughness exhibits a slight increase by 1.1–5.6 nm. The TEM characterization of the 3 at% La doped sample is shown in Fig. 3. As shown in Fig. 3a and b, the bright-field TEM images reveal that the average grain size of the sample is ∼80 nm, consistent with the SEM result (Fig. 2). The SAED pattern, as shown in Fig. 3c, exhibits diffraction rings that can be indexed to the (011), (200), (021) and (022) faces of VO2, which indicates the polycrystalline nature of the La doped VO2 thin film. The doping of La was further investigated through STEM. As shown in Fig. 3d, the atom replacement of V by La could be clearly observed in the high resolution STEM image, where the La atoms with the larger atomic number are brighter than the V atoms, arising from their different contributions to the elastic scattering signals collected by the detector. In addition, the STEM–EDX elemental mapping (Fig. 3f) related to the view of the HAADF (high angle annular dark field) shows that the La atoms are incorporated into the VO2 lattice as the distribution of La signals matches the lattice plane arrangement.
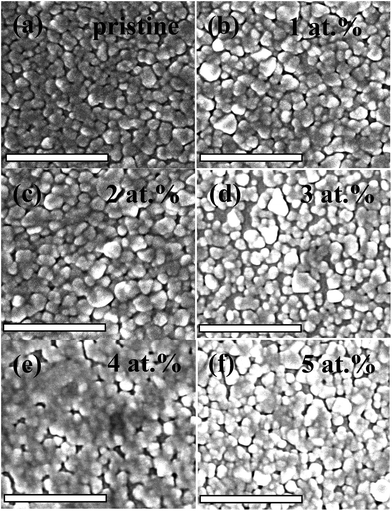 | ||
| Fig. 2 FESEM images for the pristine (a) and La doped (1–5 at%) (b–f) VO2 thin films. The scale bars in the images are 500 nm. | ||
| Doping level/at% | Thickness/nm | Raa/nm | Tlum (20 °C/90 °C)/% | ΔTsolb/% | τc (heating/cooling)/°C |
|---|---|---|---|---|---|
| a Ra is the average roughness.b ΔTsol = Tsol (20 °C) − Tsol (90 °C). | |||||
| 0 | 74 | 3.0 | 64.8/64.8 | 7.3 | 79.9/57.1 |
| 1 | 83 | 4.4 | 61.1/61.3 | 9.1 | 76.1/52.4 |
| 2 | 60 | 8.6 | 67.5/67.7 | 6.2 | 79.6/54.2 |
| 3 | 72 | 4.4 | 55.8/56.6 | 8.4 | 76.2/54.9 |
| 4 | 90 | 4.1 | 49.9/50.4 | 10.3 | 75.9/49.2 |
| 5 | 80 | 5.5 | 69.5/68.9 | 9.0 | 80.2/55.2 |
Thermochromic properties
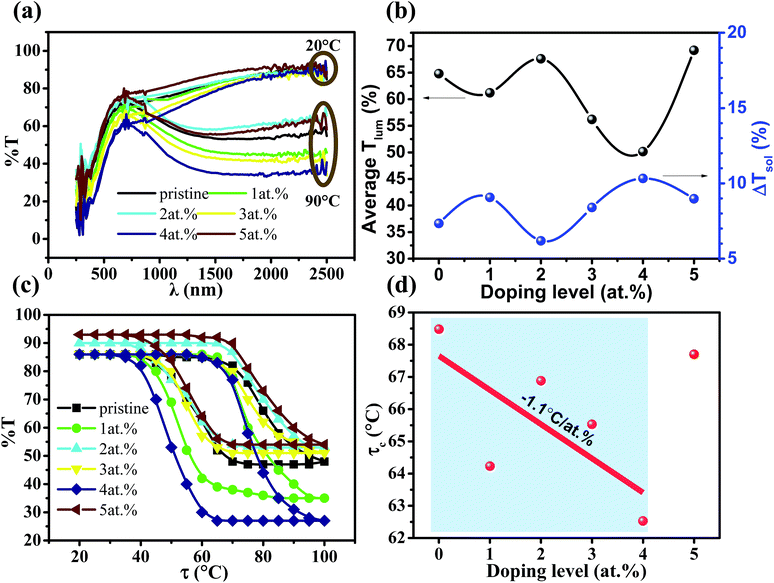
Fig. 4a shows the transmittance (% T) spectra (250 < λ < 2500 nm) of the samples at temperatures of 20 and 90 °C. Upon heating from 20 to 90 °C, a large % T contrast in the IR range could be observed for all of the samples, which should be ascribed to the MIT.20,32,33 The integrated Tlum and ΔTsol are tabulated in Table 1, and Fig. 4b shows the doping level dependent average Tlum and ΔTsol. It can be observed that the best combination of Tlum = 50.1% and ΔTsol = 10.3% was achieved at the La doping level 4 at%, which represents the best value for RE element-doped VO2 thin films,13,27,34 and is comparable to other doping cases without RE elements.35–40 It is of interest that both the average Tlum and ΔTsol at the La doping level 5 at% are higher than that of pristine VO2 (69.2%, 9.0% vs. 64.8%, 7.3%), which should be due to a surface plasmon resonance peak around 1250 nm arising from the La doping. The % T hysteresis loop was recorded at a wavelength of 2000 nm in the temperature range 20–100 °C. As shown in Fig. 4c, after doping with La, the MIT tends to move towards a lower temperature until the doping level of 4 at%, which is similar to the case with Eu doping.13 A plot of the doping level dependent τc is shown in Fig. 4d, and a τc reducing rate of −1.1 °C per at% could be attained below the doping level of 4 at%. This is the first experiment to prove the efficiency of La doping for reducing τc as indicated in simulation work using DFT calculations.28 With respect to the τc reduction mechanism, besides the lattice distortion from the substitution of large La atoms calculated by DFT,28 the increase in the hole carrier density (h+) from V5+ propagating caused by La doping (V4+1−2xV5+xLa3+xO2) should also contribute to the adjustment of τc.41 The competition between these two mechanisms should be the reason why τc starts to increase at a higher La doping level.As listed in Table 2, compared to two other reported rare earth dopants Eu3+ and Tb3+, the La3+ cation has the largest ionic radius. The average Tlum of the RE element-doped VO2 thin films decreases with the ascending ionic radius of the dopant and this is consistent with the fact that larger ions result in greater electronic polarization and slower velocity of light, leading to a larger refractive index. From Tb3+ to Eu3+ dopants, the ionic radius increases from 0.92 to 0.95 Å and the τc reducing rate is increased from 1.5 to 6.5 °C per at%, which should be due to the larger lattice distortion. However, the largest size, La3+, doping shows the lowest τc reducing rate and the reason remains unknown, but some dot defects in the form of La insertion in the seriously distorted lattice may be partially attributed to this phenomenon. In addition, compared to the previously reported W-/Mg-doped VO2 continuous thin films (Table 2), the La-doped thin film shows the best combination of Tlum (50.1%) and ΔTsol (10.3%).
| Cation | Ionic radius/Å | Doping/at% | Average Tlum/% | ΔTsol/% | dτc/dat% | Ref. |
|---|---|---|---|---|---|---|
| a The thickness, roughness and grain size of the RE element-doped VO2 thin films are at the same level. | ||||||
| RE doping | ||||||
| Eu3+ | 0.95 | 4 | 54 | 6.7 | −6.5 | 13 |
| Tb3+ | 0.92 | 4 | 65.9 | 4.6 | −1.5 | 27 |
| La3+ | 1.03 | 4 | 50.1 | 10.3 | −1.1 | This work |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Other dopings | ||||||
| W6+ | 0.6 | 2 | 45.1 | 6.9 | 20 | 42 |
| Ti4+ | 0.605 | — | 40 | 4.6 | — | 43 |
| Mg2+ | 0.72 | 5 | 82.1 | 4.8 | 1.4 | 10 |
Fig. 5a shows the % A and % R spectra of the pristine and La doped VO2 thin films. It can be found that the % A and % R could be changed by La doping, where the 5 at% and 4 at% La doped samples exhibit the lowest and highest % A/% R, resulting in the highest and lowest % T, respectively. In order to further investigate the La doping effects on the optical properties, the direct bandgap (Eg) of the VO2 thin films was calculated by fitting the linear part of the (αħω)2 vs. E curves (Fig. 5b) based on the equation (αħω)2 = A(ħω − Eg),44,45 where α is the absorption coefficient (αd = −ln(T/1 − R)), A is a constant, and ħω is the photon energy. As depicted in the inset of Fig. 5b, the Eg of the VO2 thin films gradually declines upon doping with La. The lowest value of 2.42 eV is achieved at the doping level of 3 at%, and then it returns back with further doping. The decrease of Eg below the doping level of 3 at% is similar to the case with tungsten (W) doping, which arises from the larger ionic radius,46 and the following Eg widening should be due to the competition between the effect of the large ionic radius and the h+ carrier density. Since the change in the band gap is of an electronic nature,44 the Eg narrowing of the La doped VO2 thin films below 3 at% doping should be also related to the change in the band structure resulting from the La doping in the lattice.
 | ||
| Fig. 5 (a) % A and % R spectra of the pristine and La doped (1–5 at%) VO2 thin films at room temperature. (b) Curve of (αħω)2 vs. E and the band gap Eg (inset) with different La doping levels. | ||
Conclusions
In summary, a series of La doped VO2 thin films was prepared through a facile sol–gel method, and the thin films exhibited high visible transmission and large solar modulating abilities. In particular, the 4 at% La doped sample shows the best combination of Tlum = 50.1% and ΔTsol = 10.3%, compared with other reported VO2 continuous thin films. Compared with pristine VO2, the 5 at% La doped sample can increase Tlum and ΔTsol simultaneously. La doping was first proved to be able to reduce the τc of the MIT in an experiment, which is consistent with the simulation work, and a reducing rate of −1.1 °C per at% is followed below the doping level of 4 at%. A narrowing of the band gap was observed below the doping level of 3 at%, which should be ascribed to the change in the band structure caused by the doping in the VO2 lattice with large La atoms. This investigation of La doping effects on the thermochromic properties of VO2 thin films should be useful for other element doping research using elements with a large ionic radius and low valence state.Acknowledgements
This research was supported by the Singapore National Research Foundation under the CREATE programme: Nanomaterials for Energy and Water Management, Singapore Ministry of Education (MOE) Academic Research Fund Tier 1 RG101/13, JTC-sponsored FYP program AY 2015-16 and an MSE-ERC Julich collaboration-MAP project grant. XRD, FESEM and TEM characterization was performed at the Facility for Analysis, Characterization, Testing and Simulation (FACTS) in Nanyang Technological University, Singapore and in the Ernst Ruska-Centre for Microscopy and Spectroscopy with Electrons (ER-C) in Forschungszentrum Jülich, Germany. The authors acknowledge financial support from the European Union under the Seventh Framework Programme under a contract for an Integrated Infrastructure Initiative. Reference 312483 – ESTEEM2. One of the authors, Nigel Tan Chew Shun, thanks the NTU-JTC Industrial Infrastructure Innovation Centre Singapore for their support.Notes and references
- R. Heckingbottom and J. W. Linnett, Nature, 1962, 194, 678 CrossRef CAS.
- F. Morin, Phys. Rev. Lett., 1959, 3, 34–36 CrossRef CAS.
- M. E. A. Warwick and R. Binions, J. Mater. Chem. A, 2014, 2, 3275–3292 CAS.
- Y. Gao, H. Luo, Z. Zhang, L. Kang, Z. Chen, J. Du, M. Kanehira and C. Cao, Nano Energy, 2012, 1, 221–246 CrossRef CAS.
- M. Kamalisarvestani, R. Saidur, S. Mekhilef and F. S. Javadi, Renewable Sustainable Energy Rev., 2013, 26, 353–364 CrossRef CAS.
- A. K. Prasad, S. Amirthapandian, S. Dhara, S. Dash, N. Murali and A. K. Tyagi, Sens. Actuators, B, 2014, 191, 252–256 CrossRef CAS.
- C. Liu, I. Balin, S. Magdassi, I. Abdulhalim and Y. Long, Opt. Express, 2015, 23, A124–A132 CrossRef PubMed.
- B. Hu, Y. Ding, W. Chen, D. Kulkarni, Y. Shen, V. V. Tsukruk and Z. L. Wang, Adv. Mater., 2010, 22, 5134–5139 CrossRef CAS PubMed.
- C. Wu and Y. Xie, Energy Environ. Sci., 2010, 3, 1191–1206 CAS.
- N. Wang, S. Liu, X. T. Zeng, S. Magdassi and Y. Long, J. Mater. Chem. C, 2015, 3, 6771–6777 RSC.
- X. Qian, N. Wang, Y. Li, J. Zhang, Z. Xu and Y. Long, Langmuir, 2014, 30, 10766–10771 CrossRef CAS PubMed.
- J. Cao, E. Ertekin, V. Srinivasan, W. Fan, S. Huang, H. Zheng, J. W. L. Yim, D. R. Khanal, D. F. Ogletree, J. C. Grossmanan and J. Wu, Nat. Nanotechnol., 2009, 4, 732–737 CrossRef CAS PubMed.
- X. Cao, N. Wang, S. Magdassi, D. Mandler and Y. Long, Sci. Adv. Mater., 2014, 6, 558–561 CrossRef CAS.
- A. Romanyuk, R. Steiner, L. Marot and P. Oelhafen, Sol. Energy Mater. Sol. Cells, 2007, 91, 1831–1835 CrossRef CAS.
- T. D. Manning, I. P. Parkin, C. Blackman and U. Qureshi, J. Mater. Chem., 2005, 15, 4560–4566 RSC.
- J. Du, Y. Gao, Z. Chen, L. Kang, Z. Zhang and H. Luo, Sol. Energy Mater. Sol. Cells, 2013, 110, 1–7 CrossRef CAS.
- S.-Y. Li, N. R. Mlyuka, D. Primetzhofer, A. Hallén, G. Possnert, G. A. Niklasson and C. G. Granqvist, Appl. Phys. Lett., 2013, 103, 161907 CrossRef.
- X. Cao, N. Wang, J. Y. Law, S. C. J. Loo, S. Magdassi and Y. Long, Langmuir, 2014, 30, 1710–1715 CrossRef CAS PubMed.
- M. Zhou, J. Bao, M. Tao, R. Zhu, Y. Lin, X. Zhang and Y. Xie, Chem. Commun., 2013, 49, 6021–6023 RSC.
- N. Wang, Y. Huang, S. Magdassi, D. Mandler, H. Liu and Y. Long, RSC Adv., 2013, 3, 7124–7128 RSC.
- L. Kang, Y. Gao, H. Luo, Z. Chen, J. Du and Z. Zhang, ACS Appl. Mater. Interfaces, 2011, 3, 135–138 CAS.
- A. Taylor, I. Parkin, N. Noor, C. Tummeltshammer, M. S. Brown and I. Papakonstantinou, Opt. Express, 2013, 21, A750–A764 CrossRef PubMed.
- Y. Zhou, A. Huang, Y. Li, S. Ji, Y. Gao and P. Jin, Nanoscale, 2013, 5, 9208–9213 RSC.
- X. Yuan, Y. Sun and M. Xu, J. Solid State Chem., 2012, 196, 362–366 CrossRef CAS.
- Y. Tian, J. Zhang, X. Jing and S. Xu, Spectrochim. Acta, Part A, 2012, 98, 355–358 CrossRef CAS PubMed.
- R. Sagar, P. Hudge, S. Madolappa, A. C. Kumbharkhane and R. L. Raibagkar, J. Alloys Compd., 2012, 537, 197–202 CrossRef CAS.
- N. Wang, M. Duchamp, R. E. Dunin-Borkowski, S. Liu, X. Zeng, X. Cao and Y. Long, Langmuir, 2016, 32, 759–764 CrossRef CAS PubMed.
- C. Sun, L. Yan, B. Yue, H. Liu and Y. Gao, J. Mater. Chem. C, 2014, 2, 9283–9293 RSC.
- G. Wyszecki and W. S. Stiles, Color Science: Concepts and Methods, Quantitative Data and Formulae, Wiley, New York, 2nd edn, 2000 Search PubMed.
- ASTM G173-03 Standard Tables of Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on a 37° Tilted Surface, Annual Book of ASTM Standards American Society for Testing and Materials, Philadelphia, PA, USA, 2003, vol. 14.04, http://rredc.nrel.gov/solar/spectra/am1.5 Search PubMed.
- G. Beydaghyan, V. Basque and P. V. Ashrit, Thin Solid Films, 2012, 522, 204–207 CrossRef CAS.
- N. Wang, S. Magdassi, D. Mandler and Y. Long, Thin Solid Films, 2013, 534, 594–598 CrossRef CAS.
- C. Liu, N. Wang and Y. Long, Appl. Surf. Sci., 2013, 283, 222–226 CrossRef CAS.
- L. Song, Y. Zhang, W. Huang, Q. Shi, D. Li, Y. Zhang and Y. Xu, Mater. Res. Bull., 2013, 48, 2268–2271 CrossRef CAS.
- Y. Xu, W. Huang, Q. Shi, Y. Zhang, L. Song and Y. Zhang, J. Sol-Gel Sci. Technol., 2012, 64, 493–499 CrossRef CAS.
- Y. Jiazhen, Z. Yue, H. Wanxia and T. Mingjin, Thin Solid Films, 2008, 516, 8554–8558 CrossRef.
- J. Zhang, H. He, Y. Xie and B. Pan, J. Chem. Phys., 2013, 138, 114705–114706 CrossRef PubMed.
- J. Zhou, Y. Gao, X. Liu, Z. Chen, L. Dai, C. Cao, H. Luo, M. Kanahira, C. Sun and L. Yan, Phys. Chem. Chem. Phys., 2013, 15, 7505–7511 RSC.
- Y. F. Gao, C. X. Cao, L. Dai, H. J. Luo, M. Kanehira, Y. Ding and Z. L. Wang, Energy Environ. Sci., 2012, 5, 8708–8715 CAS.
- D. Li, M. Li, J. Pan, Y. Luo, H. Wu, Y. Zhang and G. Li, ACS Appl. Mater. Interfaces, 2014, 6, 6555–6561 CAS.
- J. Wei, Z. Wang, W. Chen and D. H. Cobden, Nat. Nanotechnol., 2009, 4, 420–424 CrossRef CAS PubMed.
- L. T. Hu, H. Z. Tao, G. H. Chen, R. K. Pan, M. N. Wan, D. H. Xiong and X. J. Zhao, J. Sol-Gel Sci. Technol., 2016, 77, 85–93 CrossRef CAS.
- W. L. Hu, G. Xu, J. W. Ma, B. Xiong and J. F. Shi, Acta Phys.-Chim. Sin., 2012, 28, 1533–1538 CAS.
- S. Hu, S. Y. Li, R. Ahuja, C. G. Granqvist, K. Hermansson, G. A. Niklasson and R. H. Scheicher, Appl. Phys. Lett., 2012, 101, 201902 CrossRef.
- G.-H. Liu, X.-Y. Deng and R. Wen, J. Mater. Sci., 2010, 45, 3270–3275 CrossRef CAS.
- M. K. Dietrich, B. G. Kramm, M. Becker, B. K. Meyer, A. Polity and P. J. Klar, J. Appl. Phys., 2015, 117, 185301 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |