Synthesis of bifunctional reduced graphene oxide/CuS/Au composite nanosheets for in situ monitoring of a peroxidase-like catalytic reaction by surface-enhanced Raman spectroscopy†
Abstract
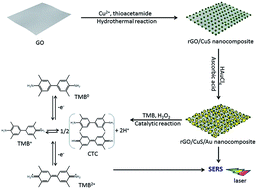
Reduced graphene oxide (rGO)/CuS/Au composite nanosheets were prepared herein via a two-step approach based on a simple hydrothermal reaction combined with an in situ reduction process. The rGO/CuS/Au composite nanosheets exhibited good peroxidase-like catalytic activity toward the oxidation of a peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of H2O2. The rGO/CuS/Au composite nanosheets were also utilized as a new surface-enhanced Raman scattering (SERS) substrate, enabling monitoring of the peroxidase-like catalytic reaction on their active surface. The oxidized intermediate of TMB formed during the peroxidase-like reaction was captured and clearly identified by SERS spectroscopy, providing an avenue for quantitative in situ monitoring of the oxidation of TMB. This approach could also be used to directly detect H2O2 with a detection limit of about 2.1 μM.
- This article is part of the themed collection: Surface enhanced Raman Spectroscopy: Editors collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...