Effect of valence states of Ni and Mn on the structural and electrochemical properties of Li1.2NixMn0.8−xO2 cathode materials for lithium-ion batteries
Shaokun Chong,
Yongning Liu*,
Wuwei Yan and
Yuanzhen Chen
State Key Laboratory for Mechanical Behavior of Materials, School of Material Science and Engineering, Xi'an Jiaotong University, Xi'an 710049, PR China. E-mail: ynliu@mail.xjtu.edu.cn; Fax: +86 29 8266 3453; Tel: +86 29 8266 4602
First published on 23rd May 2016
Abstract
Severe capacity fading and voltage decay of Li-rich layered oxides for lithium-ion batteries remain the major bottlenecks to commercialization. Herein, we studied the effect of valence states of Ni and Mn on the structure and electrochemical performances of Li1.2NixMn0.8−xO2. The oxidation states of the transition-metal (TM) were evaluated by X-ray photoelectron spectra (XPS) and validated by cyclic voltammetry (CV). With increasing Mn3+ content, the Li2MnO3 component increases due to less Li loss. The phenomenon of capacity increase upon cycling has been observed owing to the gradual activation of Li2MnO3 caused by the existence of Mn3+. It is worth mentioning that Li1.2Ni0.1Mn0.7O2 reaches the highest discharge capacity value of 231.5 mA h g−1 from the initial value of 76 mA h g−1 after 75 cycles and still keeps 100% of the capacity at 0.1C after 100 cycles. With the increase of Ni3+, Li/Ni mixing decreases because of its smaller size than Ni2+, the content of Li2MnO3 decreases as a result of the decrease of Li and Mn in the TM layers, and the discharge voltage increases on account of the higher reduction potential of Ni4+/Ni3+ compared with Ni4+/Ni2+ and Mn4+/Mn3+. Furthermore, Li1.2Ni0.4Mn0.4O2 delivers a first capacity value of 192.5 mA h g−1 and operating voltage of 3.89 V at 0.1C, and capacity retention of 92.13% and a voltage drop of 0.25 V after 100 cycles, which indicates that the stability of capacity and voltage is improved by a large margin with increasing Ni3+.
1. Introduction
Rechargeable lithium-ion secondary batteries are considered as the most valuable energy storage devices for mobile phones, laptops and electric vehicles due to high energy density, safety and long cycle life.1,2 Nowadays, the most conventional cathode materials are layered oxides LiMO2 (M = Ni, Co and Mn) with excellent cyclability, olivine LiFePO4 with low cost and long cycle life, and spinel oxides LiMn2O4 and LiNi0.5Mn1.5O4 with high voltage, while all of them suffer from low discharge capacity and fail to meet the requirements of high energy density for practical applications.3,4Recently, a layered cathode material with a chemical formula of xLi2MnO3–(1 − x)LiMO2 (M = Ni, Co and Mn) has attracted wide attention owing to its higher discharge capacity (more than 250 mA h g−1) and much higher operating voltage (about 3.5 V).5,6 However, there are many remaining problems have to be solved, such as low initial coulombic efficiency, poor rate performance, serious capacity fading and voltage decay. The low initial coulombic efficiency is associated with the release of irreversible oxygen along with the activation of Li2MnO3 during the first charge process, resulting in the formation of oxide ion vacancies.7 The poor rate performance is related to the weak intrinsic electronic conductivity resulted from insulated Li2MnO3 component and the thick solid-electrolyte interphase (SEI) layer.8 The serious capacity fading and voltage decay should be attributed to the fast layer-spinel phase transformation, which leads to a fast reduction in energy density.3,9
Li1.2Ni0.2Mn0.6O2 cathode material has become a hotspot research because of its high initial discharge capacity recently,10–13 while the problems of serious capacity fading and voltage decay caused by layered-spinel structural transformation upon cycling have not been solved radically. Thus, it is necessary to study the other cathode materials with a chemical formula of Li1.2NixMn0.8−xO2 and understand what factors affect the discharge capacity and voltage decay and further what measures should be taken to optimize the structure and ameliorate properties. It is reported that the existence of Mn3+ facilitates the activation of Li2MnO3 phase, which gives rise to a higher discharge specific capacity after the complete activation of Li2MnO3. Unfortunately, the gradual activation of Li2MnO3 induces faster layered-spinel transformation, leading to rapid capacity fading.14,15 In addition, Knight et al. have investigated the effect of Ni oxidation state on the structural and electrochemical characteristics, proposing Ni3+ can improve the cyclability and mitigate voltage decay,16 while the valence of Ni and the actual content of Li haven't been measured by any characterization methods.
Herein, we report a layered oxides with a chemical formula of Li1.2NixMn0.8−xO2 (x = 0, 0.1, 0.2, 0.3 and 0.4) prepared by a simple combustion method and a detailed study of the oxidation states of TM on the structure and electrochemical performances for the Li-rich cathode materials by changing the relative proportion of Ni and Mn. It should be noted that, the phenomenon of capacity increase upon cycling is observed by reason of the existence of Mn3+, which leads to a much higher capacity and more excellent cyclability. In addition, the stability of capacity and voltage is improved by a large margin with increasing the Ni3+. The findings herein may shed light not only on the effects understanding of the oxidation states of TM in Li-rich oxides but also on the design and improvement measures of high energy density lithium-ion batteries by controlling the content of Mn3+ and Ni3+.
2. Experimental
2.1 Synthesis of Li1.2NixMn0.8−xO2
The Li1.2NixMn0.8−xO2 (x = 0, 0.1, 0.2, 0.3 and 0.4) cathode materials were synthesized by a facile combustion method using Li, Ni, Mn acetates and citric acid as starting materials. Stoichiometric amounts of C2H3O2Li·2H2O, C4H6NiO4·4H2O and C4H6MnO4·4H2O (Sinopharm Chemical Reagent) were dissolved in ethanol under magnetic stirring to form solution A and C6H8O7·H2O (Sinopharm Chemical Reagent) was dissolved in ethanol to form solution B. A 5% excess of Li was used to offset the loss of Li during sintering under high temperature and the molar ratio of metal and citric acid was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then solution A and solution B were instilled separately into 100 ml ethanol base solution under continuous mechanical stirring. The base solution turned into white emulsion gradually as ethanol was evaporated at 80 °C and further dried at 110 °C for 12 h to obtain precursor. Finally the mixture was calcined at 450 °C for 5 h in air to remove the organics and then heated at 900 °C for 5 h in air with heating rates of 3 °C min−1.
1. Then solution A and solution B were instilled separately into 100 ml ethanol base solution under continuous mechanical stirring. The base solution turned into white emulsion gradually as ethanol was evaporated at 80 °C and further dried at 110 °C for 12 h to obtain precursor. Finally the mixture was calcined at 450 °C for 5 h in air to remove the organics and then heated at 900 °C for 5 h in air with heating rates of 3 °C min−1.
2.2 Material characterization
X-ray photoelectron spectra (XPS, Axis Ultrabld, Kratos) was used to detect the surface elemental chemical state of Li1.2NixMn0.8−xO2 (x = 0, 0.1, 0.2, 0.3 and 0.4) cathode materials. The actual elemental chemical composition of the samples was determined by a Varian 715-ES inductively coupled plasma atomic emission spectroscopy (ICP-AES). The crystal structures of prepared materials were identified by X-ray diffraction (XRD, UltimaIV-185, Rigaku) using Cu Kα radiation at 40 mA and 40 kV in the range of 10–80°.2.3 Electrochemical characterization
The electrochemical performance of Li1.2NixMn0.8−xO2 (x = 0, 0.1, 0.2, 0.3 and 0.4) cathode materials was measured on a battery test system (Neware, China) using a CR2025-type coin-cell. The cathode electrode was fabricated by coating an aluminum foil with a slurry which consisted of 80 wt% active material, 10 wt% acetylene black and 10 wt% polyvinylidene fluoride (PVDF) in N-methyl pyrrolidinone (NMP). Then the aluminum foil was dried at 100 °C for 12 h in a vacuum oven and was punched out a round cathode electrode disk of diameter 12 mm. Coin cells were assembled in an argon-filled glove box (Super 1220/750, Mikrouna) using fabricated round electrode disk as cathode, lithium foil as anode, celgard 2300 porous polypropylene film as separator and 1 M LiPF6 in EC/DMC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, vol%) as electrolyte. The galvanostatic charge/discharge was measured with a current density of 20 mA g−1 (0.1C) between 2.0 V and 4.8 V (vs. Li/Li+) at room temperature. Cyclic voltammetry (CV) was performed on a CHI750D electrochemical workstation in the potential range of 2.0–4.8 V at a scanning rate of 0.1 mV s−1.
1, vol%) as electrolyte. The galvanostatic charge/discharge was measured with a current density of 20 mA g−1 (0.1C) between 2.0 V and 4.8 V (vs. Li/Li+) at room temperature. Cyclic voltammetry (CV) was performed on a CHI750D electrochemical workstation in the potential range of 2.0–4.8 V at a scanning rate of 0.1 mV s−1.
3. Results and discussion
3.1 Structure
From chemical point of view, the Mn and Ni ions in Li1.2Ni0.2Mn0.6O2 (x = 0.2) are expected to show the +4 and +2 oxidation states, respectively.11 In order to maintain the chemical formula, the valence states of TM ions in the synthesized Li1.2NixMn0.8−xO2 are Li1.2Mn0.83+/4+O2 (x = 0), Li1.2Ni0.12+Mn0.73+/4+O2 (x = 0.1), Li1.2Ni0.32+/3+Mn0.54+O2 (x = 0.3) and Li1.2Ni0.43+Mn0.44+O2 (x = 0.4), respectively.XPS was used to determine the exact oxidation states of Mn and Ni in the layered composite material, the corresponding spectra are presented in Fig. 1. Mn2p peaks of Li1.2Mn0.8O2 (Fig. 1a), Li1.2Ni0.1Mn0.7O2 (Fig. 1b), Li1.2Ni0.2Mn0.6O2 (Fig. 1d), Li1.2Ni0.3Mn0.5O2 (Fig. 1f) and Li1.2Ni0.4Mn0.4O2 (Fig. 1h) can be fitted by multiple peaks with binding energies (BEs) (reported BEs of Mn3+ and Mn4+ are: 640.6 eV and 641.4–644.8 eV, respectively),14 the fitting results and relative percentage of Mn3+ and Mn4+ shown in Fig. 2. This means that the oxidation states of Mn for Li1.2Ni0.2Mn0.6O2, Li1.2Ni0.3Mn0.5O2 and Li1.2Ni0.4Mn0.4O2 present 4+. However, Mn ions in Li1.2Ni0.1Mn0.7O2 and Li1.2Mn0.8O2 consist of Mn3+ (40.3%) and Mn4+ (59.7%), Mn3+ (49.6%) and Mn4+ (50.4%), respectively. The existence of Mn3+ can also be proved by the oxidation peak of Mn3+/Mn4+ during initial charge process in Fig. 4(b and c). The Ni spectra are shown in Fig. 1c (Li1.2Ni0.1Mn0.7O2), Fig. 1e (Li1.2Ni0.2Mn0.6O2), Fig. 1g (Li1.2Ni0.3Mn0.5O2) and Fig. 1i (Li1.2Ni0.4Mn0.4O2). Ni2p3/2 peak fitting procedure reveals two types of BEs: 853.7–855 eV (ref. 17 and 18) and 855.3–855.9 eV.19 The lower BEs is in agreement with that of Li[Li1/3−2x/3NixMn2/3−x/3]O2 compounds, which confirms the presence of Ni2+, and the higher BEs is consistent with that of LiNiO2, which proves the presence of Ni3+. The fitting results in Fig. 2 reveal that the valence states of Ni in Li1.2Ni0.1Mn0.7O2 and Li1.2Ni0.2Mn0.6O2 are all +2. However, Ni ions in Li1.2Ni0.3Mn0.5O2 and Li1.2Ni0.4Mn0.4O2 are composed of Ni2+ (87.9%) and Ni3+ (12.1%), Ni2+ (19.9%) and Ni3+ (80.1%), respectively. Therefore, the experimentally determined oxidation states of TM ions are basically consistent with the theoretical ones.
ICP-AES analysis results of Li1.2NixMn0.8−xO2 (x = 0, 0.1, 0.2, 0.3 and 0.4) are shown in Table 1. The accurate elemental ratios of Ni/Mn are in excellent agreement with nominal values and the relative lithium content of Li1.2Ni0.2Mn0.6O2 are well close to target stoichiometries. However, the relative lithium contents of all samples decreased with increasing x value. Owing to the appearance of Ni3+ and the difficulty in maintaining Ni3+ content, more than 5% extra Li was used to compensate the Li loss during high temperature heating for Li1.2Ni0.3Mn0.5O2 and Li1.2Ni0.4Mn0.4O2.16 Furthermore, Li1.2Mn0.8O2 required little excess of Li and Li1.2Ni0.1Mn0.7O2 required less than 5% of Li to counteract the Li loss for producing the desired composition thanks to the fact that more Mn4+ was replaced by Mn3+ and there's no need to maintain the Mn4+ to increase in the degree of lithium volatilization.
| x | Sample | ICP results | a/Å | c/Å | c/a | I(003)/I(104) |
|---|---|---|---|---|---|---|
| 0 | Li1.2Mn0.8O2 | Li1.249Mn0.8O2 | 2.8647 | 14.2159 | 4.962 | 1.781 |
| 0.1 | Li1.2Ni0.1Mn0.7O2 | Li1.226Ni0.1Mn0.702O2 | 2.8737 | 14.2984 | 4.976 | 0.671 |
| 0.2 | Li1.2Ni0.2Mn0.6O2 | Li1.204Ni0.2Mn0.599O2 | 2.8676 | 14.2765 | 4.979 | 0.948 |
| 0.3 | Li1.2Ni0.3Mn0.5O2 | Li1.114Ni0.3Mn0.501O2 | 2.8779 | 14.2874 | 4.965 | 1.287 |
| 0.4 | Li1.2Ni0.4Mn0.4O2 | Li0.991Ni0.4Mn0.399O2 | 2.8799 | 14.2990 | 4.965 | 1.433 |
Fig. 3a shows the XRD patterns of Li1.2NixMn0.8−xO2 (x = 0, 0.1, 0.2, 0.3 and 0.4). The main diffraction peaks can be indexed as a hexagonal α-NaFeO2 layered structure with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group.
m space group.
 | ||
| Fig. 3 (a) XRD patterns of Li1.2NixMn0.8−xO2 (x = 0, 0.1, 0.2, 0.3 and 0.4), respectively. (b) Expanded regions of XRD patterns of all samples within a 2θ range of 20.0–22.5°, respectively. | ||
The clear splitting of (006)/(102) and (018)/(110) doublets indicate the formation of a highly ordered layered structure for all samples.20,21 The lattice parameters calculated from the XRD data are listed in Table 1. The c/a ratios of all samples are more than 4.9, which further provides the evidence for an explicit crystalline layer structure.22,23 Moreover, the ratio of I(003)/I(104) can be used to evaluate the degree of the cation mixing between Li+ and Ni2+ in the Li-layers, and the value of I(003)/I(104) > 1.2 is an indication of low degree of cation dislocation.24,25 It is indicated that there is no cation mixing in Li1.2Mn0.8O2 because the content of Ni is 0. However, the values of I(003)/I(104) for Li1.2Ni0.1Mn0.7O2 and Li1.2Ni0.2Mn0.6O2 are respectively 0.671 and 0.948, which are both lower than 1.2, indicating more cation dislocation between Li+ and Ni2+. For Li1.2Ni0.3Mn0.5O2 and Li1.2Ni0.4Mn0.4O2, the values of I(003)/I(104) are higher than 1.2, indicating lower level of cation disordering. As can be seen from above, the ratio of (003)/(104) peak increases as the Ni3+ increases because Ni3+ ions (0.56 Å) are much smaller than Ni2+ ions (0.69 Å) and Li+ ions (0.76 Å), which leads to lower Li/Ni site mixing.16
The weak peaks between 20 and 22.5° are attributed to short-range superlattice ordering of Li and Mn in the TM layers of the layered Li2MnO3 region, which can be indexed to the monoclinic unit cell with the C2/m space group.26–28 In the magnified XRD patterns (20.0–22.5°) shown in Fig. 3b, the intensity of these weak peaks appear much more intense with increasing the content of Mn, resulting from a few Li loss during high temperature heating process and more Li content in the TM layers. However, a cubic spinel structure with a space group of Fd3m can be detected in the XRD pattern (Fig. 1a) for Li1.2Mn0.8O2.
3.2 Electrochemical performance
 | ||
| Fig. 4 (a) Initial charge and discharge curves of all five samples at 0.1C between 2.0 and 4.8 V. (b–f) Corresponding cyclic voltammetry profiles. | ||
However, the capacity of sloping below 4.5 V and plateau above 4.5 V decrease as the content of Mn3+ increases, which leads to a much lower initial charge and discharge capacity for Li1.2Mn0.8O2 and Li1.2Ni0.1Mn0.7O2. For Li1.2Ni0.1Mn0.7O2, the much lower sloping capacity is most likely attributable to the serious Li/Ni site mixing. Also, the appearance of Mn3+ suppresses the complete activation of Li2MO3,14 resulting in shorter plateau above 4.5 V. The most of peaks for the CV curve of Li1.2Ni0.1Mn0.7O2 in Fig. 4c are similar to Li1.2Ni0.2Mn0.6O2, while an oxidation peak at approximately 3.25 V of Mn3+/Mn4+ is observed, which provides an evidence of Mn3+ ion formation in consistent with the result of XPS. For Li1.2Mn0.8O2, the much lower capacity can be attributed to two factors. On the one hand, the presence of Li1+xMn2−xO4 with the spinel structure leads to the diminished sloping capacity.31 On the other hand, Mn3+ and spinel structure suppress irreversible extraction of Li+ and O2− from Li2MnO3 component during the first charge process.14,31 Furthermore, the CV of Li1.2Mn0.8O2 is particularly informative. Two intense oxidation peaks of Mn3+/Mn4+ at 3.1 V and 4.25 V are observed clearly, characteristic of a Li–Mn oxide spinel phase in the system Li1+xMn2−xO4,30,32 except for the normal oxidation peak of Mn3+/Mn4+ in layer LiMnO2 at about 3.8 V and a much weaker irreversible activation of Li2MO3 at about 4.7 V during initial charge process in Fig. 4b, which is consistent with the result of XPS and XRD.
Obviously, the capacity of sloping below 4.5 V becomes higher with the increase of Ni3+ on account of much lower level of Li/Ni cation mixing. On the contrary, the capacity of plateau above 4.5 V decreases with increasing the content Ni3+, resulting in a much lower plateau capacity for Li1.2Ni0.3Mn0.5O2 and disappearance of plateau for Li1.2Ni0.4Mn0.4O2. This is most likely due to the reduction in the Li+ and Mn4+, resulting in decreasing the Li2MnO3 component.16 The CV profiles show the oxidation peaks of Ni2+/Ni3+/Ni4+ at about 4.2 V and 4.3 V for Li1.2Ni0.3Mn0.5O2 (Fig. 4e) and Li1.2Ni0.4Mn0.4O2 (Fig. 4f), respectively. However, the corresponding reduction peak splits into two separate peaks because the voltage difference between Ni3+/Ni2+ and Ni4+/Ni3+ redox couples.33 Moreover, the peak of Li2MnO3 activation can't be observed for Li1.2Ni0.4Mn0.4O2 during the initial charge process, in accordance with the disappearance of plateau and the results of XRD. In addition, it is clear that the discharge median voltage in the initial cycle increases with increasing the content of Ni3+, because Ni3+ shows the much higher redox potential compared with Ni2+ and Mn3+.
 | ||
| Fig. 5 (a) Cycling performances of all five samples at 0.1C between 2.0 V and 4.8 V. (b) Energy density curves of all the samples upon cycling at 0.1C. | ||
It is noted that Li1.2Ni0.3Mn0.5O2 and Li1.2Ni0.4Mn0.4O2 show excellent capacity retention with 75.04% and 92.13% after 100 cycles, respectively. The reason for this superior cyclic stability, which generally enhances as Ni3+ content increases, involves two aspects. One reason can be the reduction in Mn ion dissolution upon cycling resulting from decreasing the content of Mn, which leads to less capacity loss.16,35 The other reason is the reduction in Li ion irreversible dissolution and elimination of more Li vacancies upon cycling, for the smaller in size of Ni3+ than Ni2+ can't occupy the sites of Li layers after charge process, which can protect the active materials from structure transformation.16
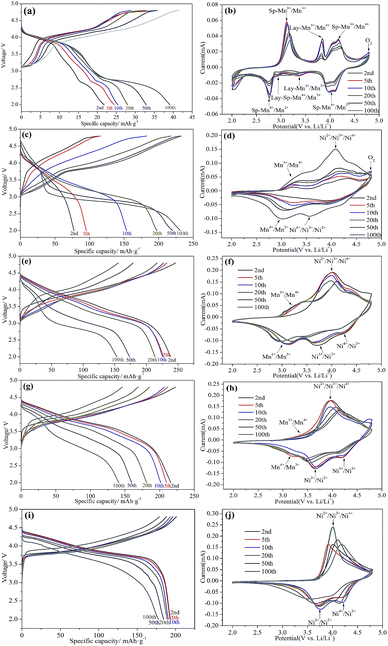
Voltage decay caused by the structure conversion from layer to spinel phase and the deterioration of the electrode/electrolyte interface is one of the main challenges for Li-rich cathode materials, which leads to the gradual decrease in energy density during cycling.36 The charge–discharge curves of all five samples at various cycle numbers at 0.1C are employed to evaluate the voltage decay, as shown in Fig. 6, respectively. Severe voltage decay upon cycling is observed in Li1.2Mn0.8O2 (Fig. 6a), Li1.2Ni0.1Mn0.7O2 (Fig. 6c) and Li1.2Ni0.2Mn0.6O2 (Fig. 6e), resulting from the unexpected layered-spinel structure transformation.37 In particular, Li1.2Mn0.8O2 exhibits an interesting gradual increase phenomenon of the discharge median voltage in the initial tens of cycles from 3.36 V to 3.60 V, for the proportion of LiMnO2 with higher redox potential of Mn3+/Mn4+ than Li1+xMn2−xO4 increases gradually along with the activation of Li2MnO3.32 However, the voltage fading rate efficiently slowed down with the increase of Ni3+ content, especially for Li1.2Ni0.4Mn0.4O2 with the voltage drop of 0.25 V. The alleviated voltage decay can be attributed to the enhancement of structural stability, the elimination of Li vacancies resulting from lower level of TM migration after charge process, the reduced Mn dissolution upon cycling and the weak interface reaction between electrode and electrolyte contributed by the reduction in oxygen release as the increase of Ni3+.16,37
Fig. 6(b, d, f, h and j) presents the CV curves of all materials after various cycles. It is obviously observed that the weak oxidation peaks of Li2O removal from Li2MnO3 at 4.8 V always exist during 100 cycles for Li1.2Mn0.8O2 (Fig. 6b), which proves the phenomenon of capacity increase upon cycling. Moreover, the oxidation peaks of Mn3+/Mn4+ in spinel phase at about 3.2 V continuously shift to a higher voltage upon cycling, and the reduction peaks of Mn4+/Mn3+ in layer phase become weaker as well as a new reduction peak of 2.95 V occurs. This behavior can be interpreted that an unexpected layered-spinel intergrowth structure formation generates voltage decay and low capacity.11 As shown in Fig. 6d, a weak peak of oxygen release still appears in the 50th during charge process and disappears after 100 cycles, which verifies the interesting phenomenon of capacity change for Li1.2Ni0.1Mn0.7O2. In particular, the redox peak of Mn4+/Mn3+ and the peak of Li2MnO3 activation cannot be observed all the time for Li1.2Ni0.4Mn0.4O2, indicating that traces of inactivated Li2MnO3 in active materials maybe enhance the structure and improve the voltage decay.
Fig. 5b shows the energy density profiles upon cycling, which are determined by capacity loss and voltage decay. Li1.2Ni0.2Mn0.6O2 delivers the highest initial energy density of 873 W h kg−1 and the lowest retention value of 446 W h kg−1 after 100 cycles. However, the gradual increase of capacity for Li1.2Ni0.1Mn0.7O2 and the excellent stability of capacity and voltage for Li1.2Ni0.4Mn0.4O2 exhibit much higher energy density of 660 W h kg−1 and 645 W h kg−1 after 100 cycles, respectively.
4. Conclusions
In this paper, a monoclinic-hexagonal layered material with a chemical formula of Li1.2NixMn0.8−xO2 (x = 0, 0.1, 0.2, 0.3 and 0.4) was prepared by a combustion method to investigate the effect of valence states of Ni and Mn on the structure and electrochemical performances. A certain amount of Mn3+ in Li-rich cathode materials can be a useful approach to obtain the higher capacity and superior cyclability after the complete activation of Li2MnO3, while it will cause serious voltage decay. For example, Li1.2Ni0.1Mn0.7O2 reaches the highest discharge capacity of 231.5 mA h g−1 from the initial value of 76 mA h g−1 after 75 cycles and still keeps 100% of the capacity at 0.1C after 100 cycles, while the operating voltage drops from 3.60 V to 2.85 V after 100 cycles. In addition, Ni3+ plays an important role in improving the stability of capacity and voltage. And operating voltage increases with the content of Ni3+ because of the higher reduction potential of Ni4+/Ni3+ compared with Ni4+/Ni2+ and Mn4+/Mn3+. But Ni3+ will give rise to a lower discharge capacity for a little oxygen release. For instance, Li1.2Ni0.4Mn0.4O2 delivers the discharge capacity of 192.5 mA h g−1 in the first cycle at 0.1C with the capacity retention of 92.13%, and initial operating voltage of 3.89 V with the voltage drop of 0.25 V after 100 cycles. Therefore, it is proposed that the amounts of Mn3+ and Ni3+ need to be well controlled during synthesis of Li-rich layered oxides to reach a reasonable integrated performance between high capacity, high operating voltage and excellent stability of capacity and voltage.Acknowledgements
This work was supported by the China Postdoctoral Science Foundation (no. 2012M521760) and the Fundamental Research Funds for the Central Universities (no. xjj2014052).References
- M. S. Whittingham, Chem. Rev., 2004, 104, 4271–4302 CrossRef CAS PubMed.
- G.-Y. Kim, S.-B. Yi, Y. J. Park and H.-G. Kim, Mater. Res. Bull., 2008, 43, 3543–3552 CrossRef CAS.
- J. Yan, X. Liu and B. Li, RSC Adv., 2014, 4, 63268–63284 RSC.
- P. R. Ilango, T. Subburaj, K. Prasanna, Y. N. Jo and C. W. Lee, Mater. Chem. Phys., 2015, 158, 45–51 CrossRef CAS.
- J. Zheng, M. Gu, A. Genc, J. Xiao, P. Xu, X. Chen, Z. Zhu, W. Zhao, L. Pullan and C. Wang, Nano Lett., 2014, 14, 2628–2635 CrossRef CAS PubMed.
- M. M. Thackeray, C. S. Johnson, J. T. Vaughey, N. Li and S. A. Hackney, J. Mater. Chem., 2005, 15, 2257–2267 RSC.
- J. Zheng, P. Xu, M. Gu, J. Xiao, N. D. Browning, P. Yan, C. Wang and J.-G. Zhang, Chem. Mater., 2015, 27, 1381–1390 CrossRef CAS.
- M. Hou, J. Liu, S. Guo, J. Yang, C. Wang and Y. Xia, Electrochem. Commun., 2014, 49, 83–87 CrossRef CAS.
- Z. Lu and J. R. Dahn, J. Electrochem. Soc., 2002, 149, A815–A822 CrossRef CAS.
- Y. Liu, Q. Wang, X. Wang, T. Wang, Y. Gao, M. Su and A. Dou, Ionics, 2015, 21, 2725–2733 CrossRef CAS.
- T. Zhao, L. Li, R. Chen, H. Wu, X. Zhang, S. Chen, M. Xie, F. Wu, J. Lu and K. Amine, Nano Energy, 2015, 15, 164–176 CrossRef CAS.
- D. Wang, Y. Huang, Z. Huo and L. Chen, Electrochim. Acta, 2013, 107, 461–466 CrossRef CAS.
- D. Wang, I. Belharouak, G. Zhou and K. Amine, Adv. Funct. Mater., 2013, 23, 1070–1075 CrossRef CAS.
- L. Xiao, J. Xiao, X. Yu, P. Yan, J. Zheng, M. Engelhard, P. Bhattacharya, C. Wang, X.-Q. Yang and J.-G. Zhang, Nano Energy, 2015, 16, 143–151 CrossRef CAS.
- X. Zhang, D. Luo, G. Li, J. Zheng, C. Yu, X. Guan, C. Fu, X. Huang and L. Li, J. Mater. Chem. A, 2013, 1, 9721–9729 CAS.
- J. C. Knight and A. Manthiram, J. Mater. Chem. A, 2015, 3, 22199–22207 CAS.
- L. Peng, Y. Zhu, U. Khakoo, D. Chen and G. Yu, Nano Energy, 2015, 17, 36–42 CrossRef CAS.
- B. Li, C. Li, J. Cai and J. Zhao, J. Mater. Chem. A, 2015, 3, 21290–21297 CAS.
- M. E. Spahr, P. Novák, B. Schnyder, O. Haas and R. Nesper, J. Electrochem. Soc., 1998, 145, 1113–1121 CrossRef CAS.
- Z.-L. Gong, H.-S. Liu, X.-J. Guo, Z.-R. Zhang and Y. Yang, J. Power Sources, 2004, 136, 139–144 CrossRef CAS.
- M. Xu, Z. Chen, H. Zhu, X. Yan, L. Jun Li and Q. Zhao, J. Mater. Chem. A, 2015, 3, 13933–13945 CAS.
- J. Lin, D. Mu, Y. Jin, B. Wu, Y. Ma and F. Wu, J. Power Sources, 2013, 230, 76–80 CrossRef CAS.
- K. Shaju, G. S. Rao and B. Chowdari, Electrochim. Acta, 2002, 48, 145–151 CrossRef CAS.
- H. Zhang, Q. Qiao, G. Li, S. Ye and X. Gao, J. Mater. Chem., 2012, 22, 13104–13109 RSC.
- B. Hwang, Y. Tsai, D. Carlier and G. Ceder, Chem. Mater., 2003, 15, 3676–3682 CrossRef CAS.
- K. A. Jarvis, Z. Deng, L. F. Allard, A. Manthiram and P. J. Ferreira, Chem. Mater., 2011, 23, 3614–3621 CrossRef CAS.
- S.-H. Kang, P. Kempgens, S. Greenbaum, A. Kropf, K. Amine and M. Thackeray, J. Mater. Chem., 2007, 17, 2069–2077 RSC.
- L. Xu, P. Hou, Y. Zhang, H. Zhang, D. Song, X. Shi, X. Wang and L. Zhang, J. Mater. Chem. A, 2015, 3, 21219–21226 CAS.
- J.-S. Kim, C. S. Johnson, J. T. Vaughey, M. M. Thackeray, S. A. Hackney, W. Yoon and C. P. Grey, Chem. Mater., 2004, 16, 1996–2006 CrossRef CAS.
- M. Thackeray, A. De Kock, M. Rossouw, D. Liles, R. Bittihn and D. Hoge, J. Electrochem. Soc., 1992, 139, 363–366 CrossRef CAS.
- D. Ye, K. Ozawa, B. Wang, D. Hulicova-Jurcakova, J. Zou, C. Sun and L. Wang, Nano Energy, 2014, 6, 92–102 CrossRef CAS.
- J. R. Croy, D. Kim, M. Balasubramanian, K. Gallagher, S.-H. Kang and M. M. Thackeray, J. Electrochem. Soc., 2012, 159, A781–A790 CrossRef CAS.
- L. Wang, G. Liu, W. Wu, D. Chen and G. Liang, J. Mater. Chem. A, 2015, 3, 19497–19506 CAS.
- L. Li, B. Song, Y. Chang, H. Xia, J. Yang, K. Lee and L. Lu, J. Power Sources, 2015, 283, 162–170 CrossRef CAS.
- S. Shi, J. Tu, Y. Zhang, Y. Zhang, C. Gu and X. Wang, Electrochim. Acta, 2013, 109, 828–834 CrossRef CAS.
- X. Liu, Q. Su, C. Zhang, T. Huang and A. Yu, ACS Sustainable Chem. Eng., 2015, 4, 255–263 CrossRef.
- B. Li, C. Li, J. Cai and J. Zhao, J. Mater. Chem. A, 2015, 8, 3308–3318 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |