Synergistic effects of hyperosmotic polymannitol based non-viral vectors and nanotopographical cues for enhanced gene delivery†
PanKaj
Garg‡
a,
Shambhavi
Pandey‡
a,
Hong-Nam
Kim
b,
Hoon
Seonwoo
a,
Sunho
Park
c,
Kyoung Soon
Choi
d,
Kyoung-Je
Jang
a,
Hoon
Hyun
e,
Phil-Hoon
Choung
*f,
Jangho
Kim
*c and
Jong Hoon
Chung
*a
aDepartment of Biosystems & Biomaterials Science and Engineering, Seoul National University, Seoul, 151-742, Republic of Korea. E-mail: jchung@snu.ac.kr
bCenter for BioMicrosystems, Brain Science Institute, Korea Institute of Science and Technology, Seoul 02792, Republic of Korea
cDepartment of Rural and Biosystems Engineering, Chonnam National University, Gwangju, 61186, Republic of Korea. E-mail: rain2000@jnu.ac.kr
dAdvanced Nano-Surface Research Group, Korea Basic Science Institute (KBSI), Daejeon 305-333, Republic of Korea
eDepartment of Biomedical Sciences, Chonnam National University Medical School, Gwangju 61469, Republic of Korea
fDepartment of Oral and Maxillofacial Surgery and Dental Research Institute, School of Dentistry, Seoul National University, Seoul 110-774, Republic of Korea. E-mail: choungph@snu.ac.kr
First published on 16th November 2016
Abstract
Here, we report the synergistic effects of hyperosmotic and nanotopographical cues designed using non-viral vectors and nanopatterned matrices for gene delivery. We show that efficiency of gene delivery can be further enhanced by two factors in combination, indicating the importance of synergistic cues in designing non-viral gene delivery platforms and strategies for gene therapy.
Gene delivery has been proposed as a potential therapy to treat various diseases through the insertion of targeted genes into cells, yielding one of the important keys in future medicine.1–3 Currently, non-viral gene vectors are commonly suggested as a suitable gene delivery system toward clinical applications due to safety concerns compared to viral gene vectors which may induce undesirable immune responses or aberrant gene expressions.4–6 To facilitate entry of the targeted genes to cells using non-viral vectors, positively charged materials, such as polyethylenimine (PEI), chitosan, and cationic liposome, condense negatively charged DNA to form nanoplexes. And then, the nanoplexes can usually be delivered into host cells through endocytosis.6 Although the non-viral gene delivery systems hold great potential, the low efficiency of gene transfection still remains a major limitation.
To overcome this considerable challenge on the use of non-viral gene vectors, the current efforts are mainly focused on the design and manipulation of new materials (e.g., synthetics of novel non-viral gene vectors).6,7 On the other hand, the recent studies suggest that gene transfection efficiency is also regulated by extracellular environments of cell culture substrates including stiffness,8,9 cell adhesion domains,10,11 and topography.12,13 For example, Leong's group reported that microscale pitted topographies enhanced the Lipofectamine-based gene transfection in fibroblasts probably due to the enhanced cell-substrate interactions.12 This pioneering study provided an important insight into the new strategies to improve the efficiency of gene transfection with non-viral gene vectors.
In conjunction with current efforts, here we propose a rational design for non-viral gene delivery platforms using the combination of hyperosmotic and nanotopographical cues, which can synergistically enhance the efficiency of gene delivery. We hypothesized that (i) the nanoplexes would be more exposed to the cells via the nanotopography-controlled cell adhesion and (ii) the efficiency of gene delivery on nanopatterned matrix could be enhanced by the chemical modification of non-viral gene vectors for the hyperosmotic cues (Fig. 1a).
We first prepared a highly aligned nanopatterned matrix and a flat substrate as a control by the ultraviolet-assisted capillary force lithography (CFL) with polyurethane acrylate.13–16 As shown in Fig. 1b, the nanogrooves and the flat patterned substrate were fabricated onto glass coverslips. It was shown that the structures were well-defined with high physical integrity and uniformity over an area of 2.5 × 2.5 cm2.
To investigate the effects of highly aligned nanotopography in terms of cell adhesion and shape, we cultured NIH3T3 fibroblasts on the substrates for 12 h. It is noted that NIH3T3 fibroblasts were obtained from ATCC® CRL-1658™, Virginia, USA. Low passage adenocarcinoma human alveolar basal epithelial (A549) were obtained from Korean cell line bank (KCLB®) of Seoul National University and culture was maintained in T-75 culture flasks (Nunc, Thermo Scientific) in 15 ml complete medium containing Roswell Park Memorial Institute (RPMI)-1640 (HyClone Laboratories, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, HyClone Laboratories, USA) and 1% antibiotic cocktail of streptomycin and penicillin. Luciferase reporter, pGL3-vector with SV-40 promoter and enhancer encoding firefly (Photonus pyralis) luciferase was obtained from Promega (Madison, WI, USA). The green fluorescent protein (GFP) gene was obtained from Clontech (Palo Alto, CA, USA). The hMSCs were isolated from human adipose tissues by the approval of the Institutional Review Board of the Ajou University of Medicine (Suwon, Korea).
The immunofluorescence staining analysis clearly showed that the cell adhesion was greatly influenced as supported by the aligned cell shape (i.e., both cytoskeletal and nucleus structure) in response to the nanotopography (Fig. 1c). To investigate the controlled cell shape on the nanopatterned matrix in further detail, the quantitative analysis was performed (Fig. 1d). Interestingly, it was found that the perimeter of cell body was much larger on the nanopatterned matrix than that on the flat substrate, whereas the nanotopography cues did not much influence the cell area. Therefore, we conjectured that the enhanced cell perimeter on the nanopatterned matrix could have an important influence on sensitivity to the gene transfection efficiency. Namely, it was expected that the nanotopography-induced cell shape could allow for effective exposure to the non-viral gene vectors.
To check the effect of nanotopographical cues on transfection efficiency, NIH3T3 fibroblasts were cultured on nanogrooves of various sizes ranging from 200 nm to 5 µm (i.e., 200 nm, 400 nm, 550 nm, 800 nm, and 5 µm) and transfected with luciferase plasmid (pGL3) using commercially available gene delivery vectors PEI25K and lipofectamine. Our results showed that the feature sizes of nanopatterns played the significant role in transfection ability of gene delivery vectors (Fig. 2). Both PEI25K/pGL3 and Lipofectamine/pGL3 were found to show higher luciferase expression as the width of nanogroove increased from 200 nm to 800 nm, almost ten times higher than the flat surfaces. On the other hand, the transfection efficiency was not much effected at 5 µm nanogroove size in comparison to the flat surface, indicating the importance of nanoscale topographical platforms for gene delivery.
Next, the interaction between nanotopographical and hyperosmotic cues on the gene transfection efficiency in NIH3T3 fibroblasts was investigated using three types of non-viral vectors as follows: Lipofectamine, PEI, and polymannitol based gene transporter (PMGT). It is noted that Lipofectamine and PEI are known as one of representative non-viral gene vectors which can form DNA complexes with cationic lipid (i.e., lipoplex) and positively charged polymer (i.e., polyplex), respectively. PMGT is a modified PEI-based non-viral gene vector that can use the hyperosmoticity contributed by polymannitol backbone leading accelerated cellular uptake and enhanced gene transfection compared to the PEI. Briefly, PMGT was synthesized in a two-step reaction in which mannitol was first esterified with methacryloyl chloride to form mannitol dimethacrylate (MDM) monomer, and then copolymerized with bPEI (1.2 kDa) by a Michael addition reaction to obtain PMGT. PMGT was prepared by same method as previously reported17 (Table S1†).
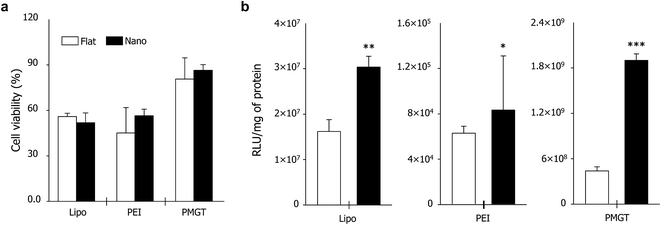
The cell viability on the systems was checked before investigating the gene transfection efficiency. We cultured NIH3T3 fibroblasts on the substrates for 12 h, and then the cells were exposed to the non-viral gene vectors for 3 h. As shown in Fig. 3a, the nanotopographical property of substrates did not much influence the cell viability. Furthermore, PMGT showed the highest biocompatibility compared to other materials in both nanopatterned matrix and flat substrate. This indicates that the suitable design of non-viral gene vectors is important in reducing the cytotoxicity.
Next, the gene transfection efficiency was investigated using PMGT/DNA and PEI/DNA polyplexes. PMGT/DNA and PEI/DNA polyplexes were characterized for particle size and zeta potential measurements using HR-TEM and dynamic light scattering (DLS). Results showed smaller size and more consistent zeta potential on PMGT/DNA polyplexes compared to the PEI/DNA complexes. The N/P ratio 20 of PMGT/DNA polyplexes and PEI/DNA complexes were used for further experiments (Fig. S1 and S2†). NIH3T3 fibroblasts were cultured primarily on the substrates for 12 h. The PMGT and PEI vectors were complexed with DNA (pGL3), and added into the culture medium. After 24 h of additional cell culture, the gene transfection was checked using luciferase assay. Two notable findings were derived from this experiment. First, regardless of the vector types, the gene transfection efficiency was much higher on the nanopatterned matrix than that on the flat substrate (Fig. 3b). In particular, when the PMGT was used, the luciferase activity of cells on the nanopatterned matrix was approximately 4.5-times higher than that on the flat substrate (Fig. S3†). Second, the property of vectors greatly influenced the gene transfection efficiency, especially in the nanopatterned matrix case (Fig. 3b). For example, when the flat substrate was used, the luciferase activity of cells transfected with PMGT was approximately 60-times higher than that with PEI. On the other hand, the nanopatterned matrix induced approximately 270-times of an increase when PMGT was used instead of PEI. Namely, we can synergistically improve the gene transfection efficiency by the use of suitable non-viral gene vectors and nanotopography-based substrates. To verify this observation again, we cultured NIH3T3 fibroblasts with PMGT/tGFP polyplexes. As shown in Fig. S4,† the highly increased green fluorescence was observed in the cells on the nanopatterned matrix than that on the flat substrate, indicating the enhanced gene transfection efficiency due to nanotopography-induced cell adhesion.
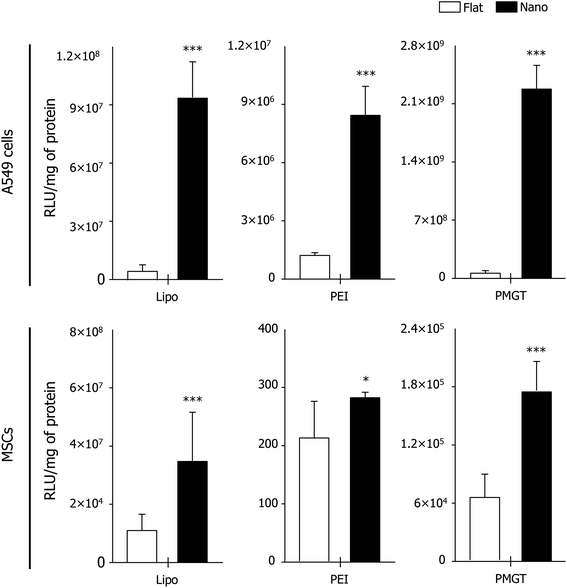
It has been widely discussed that the specialized gene delivery could be an essential strategy for cancer therapy or stem cell-based therapy.6,18,19 In this regard, designing efficient gene delivery platforms for cancer and stem cells is also one of the major issues. Thus, we expanded our strategy proposed in this study by using cancer and stem cells. To this end, we cultured human lung cancer A549 cells and human mesenchymal stem cells (MSCs) on the flat substrate and the nanopatterned matrix for 12 h, respectively. Then, the nanoplexes (i.e., pGL3 with Lipofectamine, PEI, and PMGT) were added into the culture media to investigate the effects of nanotopography and gene vectors on gene transfection efficiency in cancer and stem cells. After 24 h of culture more, we performed the luciferase assay, which clearly showed that the gene transfection efficiency was greatly enhanced on the nanopatterned matrix in both the A549 cells and MSCs, regardless of the vector types used (Fig. 4). In particular, we learned that the gene transfection efficiency in both cells was the highest when PMGT was used in synergy with the nanopatterned matrix (Fig. 4 and S3†), suggesting the importance on the use of suitable non-viral vectors (i.e., hyperosmotic polymannitol based non-viral vectors in this study) in combination with nanotopography for improving gene transfection efficiency in cancer and stem cells.
Taken together, our results indicated that the hyperosmotic and nanotopographical cues may have great potentials to enhance the gene transfection efficiency via well-designed non-viral vectors in various cell types including normal, cancer, and stem cells. Based on our experimental observations in this study, we report two notable findings (Fig. 2–4): (i) although the gene transfection efficiency could be enhanced by the nanotopographical cue, it could be further enhanced by the suitable combination of nanotopography and hyperosmotic cues (i.e., nanopatterned matrix and PMGT in this study), and (ii) the nanotopographical cue in particular promotes the transfection efficiency in cancer cells compared to that in normal or stem cells (i.e., A549 cells > NIH3T3 fibroblasts > MSCs in this study).
We discuss the following key question here: how does the nanopatterned matrix enhance the gene transfection efficiency via non-viral vectors? Although a detailed study should be performed, we propose two considerable major factors and their roles: nanotopographical and hyperosmotic cues-mediated (1) cellular shape and (2) uptake (Fig. 5).
(1) Basically, we speculated that the nanotopography-induced cellular shape would be an essential factor for the efficiency of gene delivery via non-viral gene vectors. Kong's group recently reported that the cell area of NIH3T3 fibroblasts expanded with increasing substrate stiffness, which eventually enhanced the gene transfection efficiency.9 In our study, while the aligned nanopatterned matrix did not quite influence the average of cell area, the cell perimeter was sensitively increased by the nanopatterned matrix than that on the flat substrate, meaning the elongation of cell body (Fig. 1). In addition, the nanoplexes could be located in the interstitial region between basal cell plasma membrane and nanopatterns since the plasma membranes of cells are usually suspended on such size of gaps (Fig. 5). Together, these factors collectively provided cells more opportunities to be exposed to the nanoplexes for enhancing gene delivery efficiency via non-viral gene vectors.
(2) We suggest that the highly aligned structures in both cell body and nucleus by the nanopatterned matrix (Fig. 1) would influence the cellular uptake of nanoplexes for the efficiency of gene delivery. Although the exact mechanism is unclear, it has been known that the body or nuclear shapes can modulate the exogenous gene expression.4,9,20 In this work, compared to Lipofectamine or PEI, PMGT dramatically enhanced the gene transfection on the nanopatterned matrix than on the flat substrate (Fig. 2 and 3). PMGT can lead accelerated cellular uptake than PEI or Lipofectamine due to its hyperosmotic effect which induces COX-2 expression as an inflammatory response to regain equilibrium across the cell membrane. This leads to fast cellular uptake of hyperosmotic polyplexes via caveolae-mediated endocytosis resulting in enhanced transfection.17 In addition, the transfection efficiency was much higher in the case of cancer cells rather than the normal or stem cells, regardless of types of gene vectors (Fig. 3 and 4). Therefore, the modulated cellular activity (e.g., endocytosis) by the changed cell structures on nanopatterned matrix may affect the enhanced gene delivery efficiency (Fig. 5), but further detailed studies should be performed for understanding this mechanism.
We would like to re-emphasize our findings that the highly aligned nanopatterned matrix and non-viral vectors can synergistically enhance the efficiency of gene delivery. This, therefore, suggests that there will be a further opportunity to improve gene transfection efficiency by designing efficient nanotopography-based substrates and hyperosmotic polymannitol based non-viral vectors as well as in combination. We also believe that our simple strategy may contribute to a significant progression for the design and manipulation of platforms in the various research fields. For example, this strategy could be used for enhancing stem cell functions (e.g., efficient delivery of small molecules to control stem cell differentiation (Fig. S5†) or for scaffold fabrication toward tissue regeneration).
In conclusion, we have proposed a conceptual design of platform that nanotopographical and hyperosmotic cues could promote the gene transfection efficiency. Our work suggests that the nanotopography definition of substrate and non-viral vectors should be considered in the design and manipulation of gene delivery systems for gene medicine as well as for stem cells and regenerative medicine.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2015R1D1A1A01059283, and NRF-2016M3A9B4919374). This work also supported by the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (HI15C0968).Notes and references
- D. Luo and W. Saltzman, Nat. Biotechnol., 2000, 18, 33 CrossRef CAS PubMed.
- D. Glover, H. Lipps and D. Jans, Nat. Rev. Genet., 2005, 6, 299 CrossRef CAS PubMed.
- J. Liu, Y. Li, D. Ma, D. Ouyang and Z. Xi, Chem. Commun., 2016, 52, 1953 RSC.
- A. Adler and K. Leong, Nano Today, 2010, 5, 553 CrossRef CAS PubMed.
- T. Kim, H. Jang, D. Jere, I. Parkm, M. Cho, J. Nah, Y. Choi, T. Akaike and C. Cho, Prog. Polym. Sci., 2007, 32, 726 CrossRef CAS.
- S. Pandey, P. Garg, K. Lim, J. Kim, Y. Choung, Y. Choi, P. Choung, C. Cho and J. Chung, Biomaterials, 2013, 34, 3716 CrossRef CAS PubMed.
- S. O'Rorke, M. Keeney and A. Pandit, Prog. Polym. Sci., 2010, 35, 441 CrossRef.
- H. Kong, J. Liu, K. Riddle, T. Matsumoto, K. Leach and D. Mooney, Nat. Mater., 2005, 4, 460 CrossRef CAS PubMed.
- C. Chu and H. Kong, Acta Biomater., 2012, 8, 2612 CrossRef CAS PubMed.
- H. Kim, S. Hsiong and D. Mooney, Nano Lett., 2007, 7, 161 CrossRef PubMed.
- A. Dhaliwal, M. Maldonado, Z. Han and T. Segura, Acta Biomater., 2010, 6, 3436 CrossRef CAS PubMed.
- A. Adler, A. Speidel, N. Christoforou, K. Kolind, M. Foss and K. Leong, Biomaterials, 2011, 32, 3611 CrossRef CAS PubMed.
- B. Teo, S. Goh, T. Kustandi, W. Loh, H. Low and E. Yim, Biomaterials, 2011, 32, 9866 CrossRef CAS PubMed.
- K. Suh, M. Park and P. Kim, Adv. Funct. Mater., 2009, 19, 2699e712 Search PubMed.
- J. Kim, H. Kim, K. Lim, Y. Kim, H. Seonwoo, S. Park, H. Lim, D. Kim, K. Suh, P. Choung, Y. Choung and J. Chung, Sci. Rep., 2013, 3, 03552 CrossRef PubMed.
- J. Kim, H. Kim, K. Lim, Y. Kim, S. Pandey, P. Garg, Y. Choung, P. Choung, K. Suh and J. Chung, Biomaterials, 2013, 34, 7257 CrossRef CAS PubMed.
- P. Garg, S. Pandey, B. Kang, J. Kim, K. Lim, M. Cho, T. Park, Y. Choi, P. Choung, C. Cho and J. Chung, J. Mater. Chem. B, 2014, 2, 2666 RSC.
- A. Solanki, S. Shah, P. Yin and K. Lee, Sci. Rep., 2013, 3, 01553 CrossRef PubMed.
- S. Shah, A. Solank, P. Sasmal and K. Lee, J. Am. Chem. Soc., 2013, 135, 15682 CrossRef CAS PubMed.
- T. Masters, B. Pontes, V. Viasnoff, Y. Li and N. Gauthier, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 11 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra09348e |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2016 |