A simple regenerable electrochemical aptasensor for the parallel and continuous detection of biomarkers
Abstract
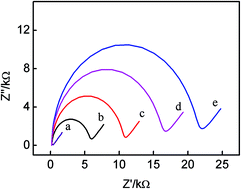
In this present work, a simple regenerable electrochemical aptasensor for the parallel and continuous detection of protein biomarkers is reported. Mucin 1 protein (MUC1) and cinoembryonic antigen (CEA) were employed as the models of protein biomarkers. The sensing interface was modified by MUC1 aptamer and methylene blue (MB)-modified CEA aptamer via hybridization with a linker DNA. Herein, MB served as a signal transducer, MUC1 and CEA were measured in parallel, and the detection limits of MUC1 and CEA were 0.13 nM and 2.75 ng mL−1, respectively. More significantly, the present biosensing interface can also be employed for the continuous detection of biomarkers. After the detection of MUC1, the resulting biosensing interface can be continuously used to measured CEA, and the detection limit was obtained as 0.5 ng mL−1, which was obviously lower than that based on the parallel analysis of CEA. Meanwhile, the aptasensor can be regenerated by rehybridization of DNA2 or DNA3-MB after the parallel detection of targets, or by introducing MUC1 and CEA and then rehybridizing the MUC1 aptamer and CEA aptamer with linker DNA1 on the gold electrode. Therefore, it can be elucidated that this present strategy provides different protocols (parallel and continuous detection) to meet the requirements of analytical applications. This present method is also cost-effective and operationally simple compared with instrument-based methods for the multiplexed detection of markers. And the accuracy, simplicity and reproducibility of this biosensor are excellent. It will give potential guidance for the multiplexed detection of biomarkers or other small molecules.
- This article is part of the themed collection: Nanoscience and nanotechnology in electrochemistry

 Please wait while we load your content...
Please wait while we load your content...