Solution-stable anisotropic carbon nanotube/graphene hybrids based on slanted columnar thin films for chemical sensing†
Abstract
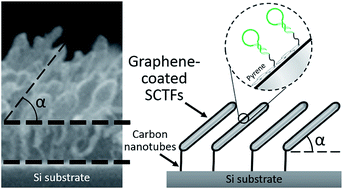
Slanted columnar thin films (SCTFs) are promising anisotropic nanostructures for applications in optical sensing and chemical separation. However, the wide use of SCTFs is significantly limited by their poor mechanical properties and structural stability, especially in liquid media. In this work, we demonstrate the fabrication of solution-stable carbon nanotube (CNT)/graphene hybrid structures based on cobalt SCTFs. The CNT/graphene hybrid structures were synthesized through the use of a titanium underlayer for Co slanted nanopillars as a chemical vapor deposition catalyst, which allows simultaneous growth of CNTs at the Co/Ti interface and three-dimensional graphene over the surface of cobalt. Importantly, the CNT/graphene hybrid structures retain the anisotropy of the parent Co SCTFs and thus remain suitable for optical sensing. Graphene/CNT modification of Co SCTFs not only improves their stability in solutions but also enables their functionalization with pyrene-modified DNA probes, which can be monitored in real time by in situ ellipsometry measurements. In turn, the solution-stable DNA-modified SCTFs may find a wide range of applications in biosensing. The described synthetic approach that allows simultaneous growth of CNTs and graphene by engineering Co/Ti interfaces may also be applied to the fabrication of other kinds of complex CNT/graphene hybrid materials.

 Please wait while we load your content...
Please wait while we load your content...