Characterization of condensed aromatics and heteroatomic species in Yanshan petroleum coke through ruthenium ion-catalyzed oxidation using three mass spectrometers†
Abstract
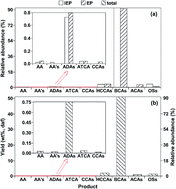
Ruthenium ion-catalyzed oxidation (RICO) of Yanshan petroleum coke (YPC) was performed to characterize condensed aromatics and heteroatomic species in YPC. The analysis with gas chromatograph/mass spectrometer mainly offers information on the distributions of side chain groups and bridged linkages, while the analyses with atmospheric pressure solid analysis probe/time-of-flight mass spectrometer and direct analysis in real time ion source coupled to time-of-flight mass spectrometer complement information on heteroatomic species and highly condensed aromatic moieties in YPC. The results indicate that YPC is rich in highly condensed aromatic moieties with tiny portions of alkyl/alkenyl side chains and alkyl/cycloalkyl bridged linkages. The organic oxygen, nitrogen, and sulfur exist in different aromatic rings (including isobenzofuran-1,3-dione, dioxoisoindoline, phenylquinoline, pyridine, and thiophene rings) and different functional groups (including methoxy, epoxy, nitro, and cyano groups). The formation of fluorine- and chlorine-containing species reveals the existence of fluorine- and chlorine-containing moieties in YPC. In addition, a series of heavier arenecarboxylic acids were produced from RICO of YPC. Their possible aromatic rings could be naphthalene, biphenyl, anthracene, phenanthrene, dihydroanthracene, dihydrophenanthrene, phenylnaphthalene, and methyl(phenyl)tetrahydroanthracene, further confirming the existence of highly condensed aromatic rings in YPC. This investigation provides an effective approach both for value-added utilization and for understanding the structural features of YPC.

 Please wait while we load your content...
Please wait while we load your content...