Synthesis of ordered meso/macroporous H3PW12O40/SiO2 and its catalytic performance in oxidative desulfurization†
Peng Yanga,
Shiyu Zhoub,
Yue Dub,
Junsheng Lib and
Jiaheng Lei*b
aSchool of Materials Science and Engineering, Wuhan University of Technology, Wuhan, Hubei 430070, China
bDepartment of Chemistry, Wuhan University of Technology, Wuhan, Hubei 430070, China. E-mail: 2187@whut.edu.cn; Tel: +86-027-8775662
First published on 27th May 2016
Abstract
Hierarchically ordered meso/macroporous phosphotungstic acid/SiO2 (HPW/SiO2) catalysts have been synthesized via the dual-templating method and used as a catalyst for oxidative desulfurization. The as-synthesized catalysts possess ordered macropores and well-ordered hexagonal mesopores within the walls of the macroporous cages. The HPW active species are finely dispersed in the silica matrix and retain its Keggin structure. Meso/macroporous HPW/SiO2 catalysts show superior catalytic performance with obvious improvement of the desulfurization rate compared to mesoporous HPW/SiO2 and macroporous HPW/SiO2. The high performance of the catalyst should be attributed to the structural characteristics of the meso/macropores. Furthermore, the catalyst was recovered and reused for seven runs with only a slight decrease in the catalytic performance.
1. Introduction
Deep desulfurization of fuels has attracted much attention due to severe environmental problems in recent years.1 Conventional hydrodesulfurization (HDS) is a widely used method in petroleum refineries. It is highly efficient in removing most sulfur compounds, but less effective for some refractory sulphur compounds, such as dibenzothiophene (DBT) and its alkyl derivatives, because of stereo hindrance.2 In order to remove these refractory sulphur compounds, the HDS process has to be performed under higher temperatures and pressures,2 which results in high operating cost. Thus, various alternative techniques such as selective adsorption, oxidative desulfurization (ODS), biodesulfurization and extraction, have been explored for deep desulfurization.3–7 The ODS, due to the advantage of mild operation conditions, is considered to be one of the most promising and economical methods.8,9 In the ODS process, the refractory sulfur compounds are oxidized to the corresponding sulfones under mild conditions, and then easily removed by extraction or adsorption.10 In recent years, heteropolyacids (HPA) with the Keggin structure are effective catalysts for ODS in fuels.11–13 However, HPA catalysts are hardly separated from the medium for reuse.14 To tackle this problem, HPA needs to be supported on suitable solid supports. It is known that mesoporous materials are excellent supports for HPA because of their high surface area and pore volume. Many researchers have proven that HPA supported on mesoporous materials exhibited a high desulfurization activity and excellent recyclability in the ODS process.13–19Hierarchically meso/macroporous materials are of great interest as catalysts or catalyst supports in recent years, because such bi-modal pore structures are especially beneficial for the mass transport in heterogeneous catalytic reactions and the sufficient exposure of active catalytic sites.20,21 Previous studies have demonstrated that hierarchically meso/macroporous catalysts show superior catalytic performance. As reported by Huang et al., the Co–Mo–Ni/Al2O3 catalyst with meso/macroporous structure exhibited much higher hydrodesulfurization activity than mesoporous catalyst.22 Han et al. synthesized a meso/macroporous alumina supported Co–Mo catalyst, and found that this catalyst displayed higher desulfurization rate compared to mesoporous catalyst.23 Parkhomchuk et al. prepared a Co–Mo catalyst supported on meso/macroporous alumina, which showed higher activity for hydrodesulfurization of heavy oil than conventional catalyst with only a mesoporous structure.24 Consequently, it is believe that hierarchically meso/macroporous system should be beneficial for catalytic oxidative desulfurization.
In this work, Keggin-type HPW were incorporated into the ordered meso/macroporous silica framework to enhance the desulfurization performance and reusability of the catalyst. The effects of HPW loading amount, catalyst dosage, oxidant amount, and reaction temperature on the desulfurization activity of the catalysts were systematically studied. In order to investigate the effect of porous structure on the catalytic activity, macroporous catalyst and mesoporous catalyst were used as references. Moreover, the reusability of the catalyst was investigated as well.
2. Experimental
2.1. Chemicals
Model compounds (Scheme 1) and chemicals, including dimethyldibenzothiophene (DBT), benzothiophene (BT), 4,6-dimethyldibenzothiophene (4,6-DMDBT), phosphotungstic acid (HPW), tetramethyl orthosilicate (TMOS) and Pluronic P123 (EO20PO70EO20), were purchased from Sigma-Aldrich. Styrene, potassium persulfate, sodium hydroxide, hydrochloric acid, acetonitrile, petroleum ether (boiling range: 90–120 °C) and 30 wt% H2O2 were purchased from Sinopharm Chemical Reagent Co., Ltd. All chemicals were used for experiments without any further purification.2.2. Catalyst preparation
Monodisperse polystyrene (PS) microspheres with a mean size of ∼400 nm were synthesized via an emulsifier-free emulsion polymerization approach.25 A 250 mL round-bottomed four-necked flask was charged with 120 mL of water and 8 mL of styrene (pre-washed 3 times with 2 M NaOH, then 4 times with distilled water), and then heated to 70 °C under N2 atmosphere. 0.14 g of K2S2O8 was added to the reaction mixture, and then kept at 70 °C for 6 h. The PS colloidal crystal template was assembled via a centrifugation method. The resulting emulsion was centrifuged at 3000 rpm for 10 h, and then the supernatant of the mixture was removed using a dropper. The solid block was dried at room temperature to obtain the PS colloidal crystal template (Fig. S1†). A typical procedure for synthesizing the hierarchically ordered meso/macroporous HPW/SiO2 was carried out as follows: 1.0 g of P123 and a certain amount of HPW were dissolved in 4 mL of 0.05 M HCl solution with stirring at 35 °C. Then, 2.0 g of TMOS was added gradually under vigorous stirring, and maintained the stirring for 10 min. Subsequently, the precursor solution was added into a beaker containing 2–3 pieces of PS template, while maintained at 35 °C for 24 h. The composite monolith was then carefully scratched with a blade to remove extra gel precursor from their surface, and dried at 100 °C. Finally, the solid product was calcined in air at 400 °C for 15 h (ramping rate of 1 °C min−1) to remove PS colloidal crystal and P123 templates. The obtained meso/macroporous HPW/SiO2 catalyst was denoted as m/M-HPW/SiO2-x, where x stands for the weight percentage of HPW in the catalyst.For comparison purpose, macroporous HPW/SiO2 and mesoporous HPW/SiO2 catalysts with 20 wt% HPW content were prepared, respectively. The order macroporous HPW/SiO2 was synthesized following the similar procedure described above but without the addition of the P123 template. The order mesoporous HPW/SiO2 catalyst was synthesized according to the literature reported previously.15 The obtained macroporous HPW/SiO2 and mesoporous HPW/SiO2 catalysts were designated as M-HPW/SiO2-20 and m-HPW/SiO2-20, respectively.
2.3. Catalyst characterization
Scanning electron microscope (SEM) images were taken with a Hitachi S-4800 field emission scanning electron microscope. Transmission electron microscopy (TEM) images were obtained with a JEOL JEM-2100F electron microscope operating at 200 kV. Nitrogen sorption isotherms were measured with a Micromeritics Tristar II 3020 analyzer at 77 K. The specific surface areas were calculated by the Brunauer–Emmett–Teller (BET) method. The total pore volumes were calculated from the basis of the amount adsorbed at a relative pressure of ∼0.99. The pore size distributions were derived from the adsorption branches of isotherms using the Barrett–Joyner–Halenda method. X-ray diffraction (XRD) measurements were performed in a Bruker D8 Advance diffractometer with a CuKα radiation (λ = 1.5406 Å). Fourier transform infrared spectra (FT-IR) were performed in a Digilab-FTS60 spectrometer. The HPW content in catalyst sample was determined by the inductively coupled plasma (ICP) analysis on a Perkin-Elmer 3300DV.2.4. Oxidative desulfurization of model fuel
The model fuels with sulfur content of 500 mg L−1 (S) were obtained by dissolving DBT, BT or 4,6-DMDBT in petroleum ether, respectively. The catalytic performance was performed in a 50 mL two-neck flask equipped with a water-circulated column. The reactor was immersed in a water bath to maintain the reaction temperature constant. 10 mL of model oil, 10 mL of acetonitrile and a certain amount of catalyst were added into the two-neck flask and then heated to the desired temperature. Subsequently, 30 wt% aqueous H2O2 was added into the mixture to start the reaction. The amount of oxidant was expressed as O/S (the molar ratio of H2O2 to sulfur). The solid catalyst was recovered by centrifugation, washed with methanol, dried at 100 °C, and subjected to the next ODS process. The upper model oil was collected and the sulfur content was analyzed by a high performance liquid chromatography (HPLC). The HPLC system was LC-20A (Shimadzu, Japan), which consists of LC-20AT pumps, a SPD 20A ultraviolet detector and a SinoChrom ODS-BP column (4.6 mm × 200 mm, 5 μm).3. Results and discussion
3.1. Characterization of the catalyst
A series of meso/macroporous HPW/SiO2 samples were first prepared and the morphology and porous structure of these samples were examined using SEM and TEM. The representative SEM images of m/M-HPW/SiO2 with different HPW contents are presented Fig. 1. All the samples exhibit a three-dimensionally ordered macroporous structure and interconnecting windows between the adjacent pores (Fig. 1a–c). The macropore size and window size are about 270 nm and 80 nm, respectively. Compared to the average size of the PS microspheres (Fig. S1†), the size of macropore exhibits the shrinkage of 32% due to the shrinkage of the PS microspheres and condensation of silica framework during the calcination process.26,27 Fig. 1d further reveals that the ordered macroporous structure extends throughout the entire m/M-HPW/SiO2-20 sample. TEM image in Fig. 2 clearly displays ordered macropores and mesopore networks permeating throughout the whole m/M-HPW/SiO2-20 sample. The striped pattern (Fig. 2b), which viewed perpendicular to the direction of the pore channels, confirms the periodic mesopores with hexagonal symmetry (P6mm). The mesopore size is roughly estimated to be about 5 nm from the TEM image. Combining the SEM and TEM results, they prove that m/M-HPW/SiO2 samples possess ordered meso/macroporous structure, which may be of great help for improving the desulfurization reaction.The textural properties of the meso/macroporous HPW/SiO2 samples were further analyzed by N2 sorption measurement. The isotherms of these m/M-HPW/SiO2 samples exhibit similar type IV curves with H2 hysteresis loop (Fig. 3A), demonstrating the presence of a large number of mesopores.28 The sharp capillary condensation steps occur at the region of P/Po = 0.4–0.7, suggesting a uniform mesopore. Besides the mesopores, these samples display the existence of macropores as evidenced by the appearance of a high adsorption at a high relative pressure (P/Po > 0.9).29 The pore size distribution curves were derived from the adsorption branches using the BJH model. It is revealed that these samples display a narrow pore size distributions with mean pore size ranging from 4.8 nm to 5.1 nm (Fig. 3B), in accordance with TEM result. The specific surface areas and the pore structure parameters of the meso/macroporous HPW/SiO2 samples are listed in Table 1. The surface area and pore volume of meso/macroporous HPW/SiO2 samples decrease slightly with increasing the HPW contents, which could be due to the partial collapse of the mesopore structure caused by excess HPW.17
 | ||
| Fig. 3 Nitrogen adsorption/desorption isotherms (A) and corresponding BJH pore size distribution curves (B) of m/M-HPW/SiO2 samples with different HPW contents. | ||
| Catalysts | HPW dosage (%) | SBETa (m2 g−1) | Db (nm) | Vtc (cm3 g−1) | Sulfur removald |
|---|---|---|---|---|---|
| a SBET: BET surface areas.b D: average pore size.c Vt: total pore volumes.d Reaction conditions: catalyst dosage = 0.1 g, T = 60 °C, O/S = 12 and t = 1 h. | |||||
| m/M-HPW/SiO2-10 | 9.4 | 358 | 5.0 | 0.45 | 82.0% |
| m/M-HPW/SiO2-20 | 19.5 | 346 | 5.1 | 0.44 | 97.0% |
| m/M-HPW/SiO2-30 | 29.1 | 321 | 4.8 | 0.40 | 98.8% |
| m-HPW/SiO2-20 | 19.8 | 398 | 4.4 | 0.39 | 88.2% |
| M-HPW/SiO2-20 | 19.5 | 217 | 8.1 | 0.41 | 87.8% |
The mesopore structure of the meso/macroporous HPW/SiO2 was characterized by small-angle XRD (Fig. 4A). The XRD patterns of m/M-HPW/SiO2-10 and m/M-HPW/SiO2-20 show a well-resolved diffraction peak in the small angle range, indicating the presence of ordered mesopores, which is consistent with the TEM result. When HPW content in sample increases to 30 wt%, the diffraction peak of meso/macroporous HPW/SiO2 sample almost disappears, suggesting the deterioration of the mesoporous ordering. The wide-angle XRD patterns of m/M-HPW/SiO2 samples are presented in Fig. 4B. All the samples show a strong broad peak at 24.3° and a very weak one at 43.6°, which are assigned to amorphous silica.30 The absence of HPW peaks in these samples demonstrates that HPW clusters are dispersed homogeneously in the silica framework and the increase of HPW content does not affect the dispersion level.17 The HPW contents in meso/macroporous HPW/SiO2 samples were determined using ICP elemental analysis, which are close to the expected values (Table 1). These results suggest that the HPW is successfully embedded in a silica matrix.
 | ||
| Fig. 4 Small-angle (A) and wide-angle (B) XRD patterns of (a) m/M-HPW/SiO2-10, (b) m/M-HPW/SiO2-20 and (c) m/M-HPW/SiO2-30. | ||
To study the structure of HPW in these m/M-HPW/SiO2 samples, FT-IR analysis was performed. As shown in Fig. 5, the HPW Keggin structure shows several strong bands at 1079, 987, 887 and 809 cm−1, which are usually attributed to νas(P–O) in the central PO4 tetrahedron, νas(W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the exterior WO6 octahedron, νas(W–Ob–W) in corner shared octahedral, and νas(W–Oc–W) in edge shared octahedral,31 respectively. These m/M-HPW/SiO2 samples show two weak bands of HPW at 899 and 984 cm−1, indicating that the Keggin structures of HPW remain intact after the formation of the composites. Other bands of HPW are not clearly visible in meso/macroporous HPW/SiO2 samples due to the overlapping with bonds of silica.32 The bands for W–Ob–W and W
O) in the exterior WO6 octahedron, νas(W–Ob–W) in corner shared octahedral, and νas(W–Oc–W) in edge shared octahedral,31 respectively. These m/M-HPW/SiO2 samples show two weak bands of HPW at 899 and 984 cm−1, indicating that the Keggin structures of HPW remain intact after the formation of the composites. Other bands of HPW are not clearly visible in meso/macroporous HPW/SiO2 samples due to the overlapping with bonds of silica.32 The bands for W–Ob–W and W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O show small shifts compared to that in the spectrum of bulk HPW, probably because of the chemical interaction between the HPW anion and silica.33
O show small shifts compared to that in the spectrum of bulk HPW, probably because of the chemical interaction between the HPW anion and silica.33
 | ||
| Fig. 5 FT-IR spectra of (a) SiO2, (b) m/M-HPW/SiO2-10, (c) m/M-HPW/SiO2-20, (d) m/M-HPW/SiO2-30 and (e) HPW. | ||
3.2. Effect of different parameters on sulfur removal
The meso/macroporous HPW/SiO2 samples were used as catalysts to remove the sulfur compounds in model fuels. We first evaluated the catalytic performance of the m/M-HPW/SiO2 samples with different HPW contents (Fig. 6). It is clear that the desulfurization rates of m/M-HPW/SiO2-20 and m/M-HPW/SiO2-30 are better than that of the m/M-HPW/SiO2-10, indicating that the catalytic activity enhances with the increase of the HPW content. This is because the catalytic active species per unit surface area increase with increasing HPW content. However, m/M-HPW/SiO2-30 shows approximately the same catalytic activity as m/M-HPW/SiO2-20. This may be due to the partial collapse of mesoporous structure for m/M-HPW/SiO2-30. Therefore, further experiments were carried out using m/M-HPW/SiO2-20. | ||
| Fig. 6 The desulfurization rate of meso/macroporous HPW/SiO2 with different HPW contents, catalyst dosage = 0.1 g, T = 60 °C and O/S = 12. | ||
Reaction conditions, including catalyst dosage, O/S molar ratio and reaction temperature, were investigated to obtain the optimal operating conditions with m/M-HPW/SiO2-20 catalyst. The effect of catalyst dosage on the desulfurization rate is presented in Fig. 7A. The desulfurization rate increases dramatically from 71.3% to 100% with the increase of the catalyst dosage from 0.02 to 0.1 g, due to the increase in total amount of catalytic active sites. When the catalyst dosage is further increased to 0.2 g, the desulfurization rate remains unchanged appreciably. Apparently, catalyst dosage of 0.1 g provides catalytic active sites enough to oxidative reaction.
 | ||
| Fig. 7 The effect of the (A) catalyst dosage, (B) O/S molar ratio and (C) reaction temperature on the desulfurization rate, catalyst dosage = 0.1 g, T = 60 °C, O/S = 12 and t = 2 h. | ||
Fig. 7B illustrates the effect of O/S molar ratio on the catalytic activity. With the increase of the O/S molar ratio, the desulfurization rate increases firstly and then decreases slightly. When O/S molar ratio increases from 2 to 12, the desulfurization rate is gradually enhanced. The reason may be that the increase of O/S molar ratio could improve the production of more active intermediate species,34 and thus promote the catalytic activity. Nevertheless, the sulfur removal slightly decreased when the O/S molar ratio reached up to 20. The reason may be that the surface area of catalyst is occupied by the excess aqueous solution, which affects sulfur compounds adsorption to surface area of catalyst.8 Therefore, this optimal value of O/S molar ratio was 12.
As shown in Fig. 7C, the desulfurization rate increases dramatically with the increase of reaction temperature from 40 to 60 °C. However, when the reaction temperature is over 80 °C, the catalytic activity slightly decreases. This is probably because H2O2 decomposes dramatically at high temperature, and it would result in the insufficient of available H2O2 in reaction system.12 In consideration of the energy consumption and catalytic efficiency, the reaction temperature employed for the consequent experiments was set at 60 °C. It is noteworthy that the oxidative desulfurization should avoid to be performed at high O/S molar ratio and temperature, because they would lead to a reduction of the desulfurization rate. The sulfur removal of m/M-HPW/SiO2-20 catalyst reaches up to 100% for the model fuels under the optimal conditions. Under the similar reaction conditions, the catalytic activity of meso/macroporous HPW/SiO2 is higher than those of HPW/SiO2 catalyst reported in previous literature. For example, Li and coworkers reported the conversion of DBT was lower 98.6% using HPW/mesoporous silica pillared clay as catalysts.35,36 Tang and coworkers reported sulfur removal of HPW/TUD-1 catalyst was 98.1%.37
The oxidative activities of different sulfur compounds were also evaluated in the oxidation catalyzed by m/M-HPW/SiO2-20. It is obvious that the oxidation reactivity decreased in the order of DBT > BT > 4,6-DMDBT under optimal reaction conditions (Fig. 8), the same order as observed in the biphasic catalytic system.13 The electron densities on the sulfur atoms in BT, DBT and 4,6-DMDBT are 5.739, 5.758 and 5.760,38 respectively. The BT displays the low reactivity, which is related to its lowest electron density. The electron density for 4,6-DMDBT is the highest, but catalytic activity is the lowest due to the steric hindrance by methyl groups at 4 and 6 positions of the 4,6-DMDBT molecule.34
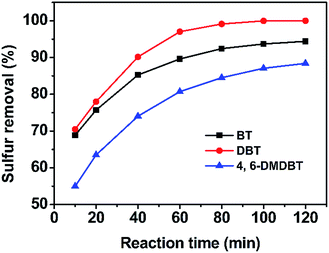 | ||
| Fig. 8 Effect of different sulfur-containing compounds on the sulfur removal, catalyst dosage = 0.1 g, T = 60 °C and O/S = 12. | ||
3.3. Influence of different catalysts on sulfur removal
In order to investigate the effect of hierarchical structure on the ODS performance, mesoporous HPW/SiO2 and macroporous HPW/SiO2 catalysts were also prepared and tested as references. The m-HPW/SiO2-20 catalyst has similar ordered mesostructure and mesopore size distribution to m/M-HPW/SiO2-20, but the spherical macropore is absent in m-HPW/SiO2-20 catalyst (Fig. S2a and S3†). Fig. 9 shows the desulfurization rates of the m-HPW/SiO2-20, M-HPW/SiO2-20 and m/M-HPW/SiO2-20 catalysts under same reaction condition, and the detailed results are summarized in Table 1. The desulfurization rate of m/M-HPW/SiO2-20 is higher than that of the m-HPW/SiO2-20. The result indicates that the macroporous channel is an important factor influencing the oxidative desulfurization reaction. The catalytic reactions are most effective when the pathway for the mass transport through which reactants and products move into or out of the nanostructured material are included as an integral part of the nanostructure.39 The transport of small molecules in media large mesopores (>10 nm) and macropores (>50 nm) can reach rate of diffusion similar to those in open medium.39 Therefore, it is believe that the interconnected macropores of m/M-HPW/SiO2-20 can serve as efficient transport channels for reactants and products, and thus improve its catalytic activity by facilitating the mass transfer. | ||
| Fig. 9 The catalytic performance of M-HPW/SiO2-20, m-HPW/SiO2-20 and m/M-HPW/SiO2-20, catalyst dosage = 0.1 g, T = 60 °C and O/S = 12. | ||
On the other hand, M-HPW/SiO2-20 and m/M-HPW/SiO2-20 catalysts possess similar ordered macroporous structures (Fig. S2b†), but the M-HPW/SiO2-20 have much lower mesoporosity and surface area than m/M-HPW/SiO2-20 (Fig. S3†). As can be seen from Fig. 9, the m/M-HPW/SiO2-20 exhibits better desulfurization performance than the M-HPW/SiO2-20. This is because that the high surface area of m/M-HPW/SiO2-20 can provide more active sites, which is advantageous for catalytic reaction.40,41 Based on the above results, it is reasonable to suggest that the excellent catalytic performance of m/M-HPW/SiO2-20 is attributed to the combination of both the positive effect of the macrochannels on mass transfer and the sufficient amount of catalytic active sites related to high surface area.
3.4. Reusability performance
The recyclability of the catalyst is one of the critical factors for industrial application. The m/M-HPW/SiO2-20 catalyst was recovered at the end of reaction by centrifugation, washed with methanol, dried at 100 °C, and then was reused under the same conditions. The desulfurization rate of the catalyst decreases from 100% to 96.2% after seven cycles (Fig. 10). The result indicates that the catalyst shows a good recyclability in the ODS process. To evaluate the stability of the catalyst, the HPW content of the catalyst was detected by ICP analysis after seven consecutive reaction cycles. The content of HPW decreases from 19.5% to 17.3%, suggesting the presence of a strong interaction between the HPW specie and the silica, which has been proved by the FT-IR spectra. Therefore, it is not surprising that the meso/macroporous HPW/SiO2 catalyst has excellent recyclability. | ||
| Fig. 10 Recycle performance of the m/M-HPW/SiO2-20 catalyst, catalyst dosage = 0.1 g, T = 60 °C, O/S = 12 and t = 2 h. | ||
4. Conclusions
Ordered meso/macroporous HPW/SiO2 catalysts have been successfully synthesized using the block copolymer P123 and polystyrene colloidal crystal as templates. The as-synthesized catalysts show hexagonally ordered mesopores and periodic interconnected macropores. The HPW with Keggin structure were embedded into the silica matrix uniformly. The meso/macroporous HPW/SiO2 catalysts exhibit good catalytic activity and excellent recyclability in oxidative desulfurization. Under the optimal reaction condition, the sulfur compound is able to be completely removed, and desulfurization rate still reach 96.2% after seven recycles. The oxidative efficiency of different sulfur compounds decrease in the order of DBT > BT > 4,6-DMDBT. Compared with macroporous HPW/SiO2 and mesoporous HPW/SiO2 catalysts, the meso/macroporous HPW/SiO2 catalyst shows higher catalytic activity in oxidative desulfurization. This is attributed to face that the macrochannel of meso/macroporous HPW/SiO2 catalyst facilitates mass transfer, and the high surface area provides the sufficient amount of active sites.Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (no. 21476177).References
- F. S. Mjalli, O. U. Ahmed, T. Al-Wahaibi, Y. Al-Wahaibi and I. M. AlNashef, Rev. Chem. Eng., 2014, 30, 337–378 CrossRef CAS.
- B. Pawelec, R. M. Navarro, J. Miguel Campos-Martin and J. L. G. Fierro, Catal. Sci. Technol., 2011, 1, 23–42 CAS.
- R. T. Bachmann, A. C. Johnson and R. G. J. Edyvean, Int. Biodeterior. Biodegrad., 2014, 86, 225–237 CrossRef CAS.
- A. A. Nuhu, Rev. Environ. Sci. Bio/Technol., 2013, 12, 9–23 CrossRef CAS.
- Y. Shen, P. Li, X. Xu and H. Liu, RSC Adv., 2012, 2, 1700–1711 RSC.
- W. Ding, W. Zhu, J. Xiong, L. Yang, A. Wei, M. Zhang and H. Li, Chem. Eng. J., 2015, 266, 213–221 CrossRef CAS.
- L. Li, J. Zhang, C. Shen, Y. Wang and G. Luo, Fuel, 2016, 167, 9–16 CrossRef CAS.
- C. Shen, Y. J. Wang, J. H. Xu and G. S. Luo, Green Chem., 2016, 18, 771–781 RSC.
- X. M. Yan, P. Mei, L. Xiong, L. Gao, Q. F. Yang and L. J. Gong, Catal. Sci. Technol., 2013, 3, 1985–1992 CAS.
- M. T. Timko, J. A. Wang, J. Burgess, P. Kracke, L. Gonzalez, C. Jaye and D. A. Fischer, Fuel, 2016, 163, 223–231 CrossRef CAS.
- J. Chen, C. Chen, R. Zhang, L. Guo, L. Hua, A. Chen, Y. Xiu, X. Liu and Z. Hou, RSC Adv., 2015, 5, 25904–25910 RSC.
- J. Zhang, A. J. Wang, X. Li and X. H. Ma, J. Catal., 2011, 279, 269–275 CrossRef CAS.
- J. Xiong, W. Zhu, W. Ding, L. Yang, M. Zhang, W. Jiang, Z. Zhao and H. Li, RSC Adv., 2015, 5, 16847–16855 RSC.
- G. Q. Luo, L. H. Kang, M. Y. Zhu and B. Dai, Fuel Process. Technol., 2014, 118, 20–27 CrossRef CAS.
- X. Yan, J. Lei, D. Liu, Y. Wu and W. Liu, Mater. Res. Bull., 2007, 42, 1905–1913 CrossRef CAS.
- J. H. Lei, L. N. Chen, P. Yang, X. D. Du and X. M. Yan, J. Porous Mater., 2013, 5, 1379–1385 CrossRef.
- J. H. Qiu, G. H. Wang, Y. Q. Zhang, D. L. Zeng and Y. Chen, Fuel, 2015, 147, 195–202 CrossRef CAS.
- Z. Abdalla and B. S. Li, Chem. Eng. J., 2012, 200, 113–121 CrossRef.
- B. Li, W. Ma, J. Liu, C. Han, S. Zuo and X. Li, Catal. Commun., 2011, 13, 101–105 CrossRef CAS.
- C. M. Parlett, K. Wilson and A. F. Lee, Chem. Soc. Rev., 2013, 42, 3876–3893 RSC.
- A. Stein, B. E. Wilson and S. G. Rudisill, Chem. Soc. Rev., 2013, 42, 2763–2803 RSC.
- Y. Huang, Z. Zhou, Y. Qi, X. Li, Z. Cheng and W. Yuan, Chem. Eng. J., 2011, 172, 444–451 CrossRef CAS.
- D. Z. Han, X. Li, L. Zhang, Y. H. Wang, Z. F. Yan and S. M. Liu, Microporous Mesoporous Mater., 2012, 158, 1–6 CrossRef CAS.
- E. V. Parkhomchuk, A. I. Lysikov, A. G. Okunev, P. D. Parunin, V. S. Semeikina, A. B. Ayupov, V. A. Trunova and V. N. Parmon, Ind. Eng. Chem. Res., 2013, 52, 17117–17125 CrossRef CAS.
- B. T. Holland, C. F. Blanford, T. Do and A. Stein, Chem. Mater., 1999, 11, 795–805 CrossRef CAS.
- F. Li, Z. Wang, N. S. Ergang, C. A. Fyfe and A. Stein, Langmuir, 2007, 23, 3996–4004 CrossRef CAS PubMed.
- J. Liu, M. Li, J. Wang, Y. Song, L. Jiang, T. Murakami and A. Fujishima, Environ. Sci. Technol., 2009, 43, 9425–9431 CrossRef CAS PubMed.
- Z. Sun, Y. Deng, J. Wei, D. Gu, B. Tu and D. Zhao, Chem. Mater., 2011, 23, 2176–2184 CrossRef CAS.
- J. Dhainaut, J. Dacquin, A. F. Lee and K. Wilson, Green Chem., 2010, 12, 296–303 RSC.
- B. Liu and R. T. Baker, J. Mater. Chem., 2008, 18, 5200–5207 RSC.
- X. M. Yan, P. Mei, J. H. Lei, Y. Z. Mi, L. Xiong and L. P. Guo, J. Mol. Catal. A: Chem., 2009, 304, 52–57 CrossRef CAS.
- A. Bordoloi, F. Lefebvre and S. B. Halligudi, J. Catal., 2007, 247, 166–175 CrossRef CAS.
- I. V. Kozhevnikov, Chem. Rev., 1997, 98, 171–198 CrossRef.
- M. Te, C. Fairbridge and Z. Ring, Appl. Catal., A, 2001, 219, 267–280 CrossRef CAS.
- B. Li, Z. Liu, J. Liu, Z. Zhou, X. Gao, X. Pang and H. Sheng, J. Colloid Interface Sci., 2011, 362, 450–456 CrossRef CAS PubMed.
- B. Li, Z. Liu, C. Han, W. Ma and S. Zhao, J. Colloid Interface Sci., 2012, 377, 334–341 CrossRef CAS PubMed.
- L. Tang, G. Luo, M. Zhu, L. Kang and B. Dai, J. Ind. Eng. Chem., 2013, 19, 620–626 CrossRef CAS.
- S. Otsuki, T. Nonaka, N. Takashima, W. H. Qian, A. Ishihara, T. Imai and T. Kabe, Energy Fuels, 2000, 14, 1232–1239 CrossRef CAS.
- D. R. Rolison, Science, 2003, 299, 1698–1701 CrossRef CAS PubMed.
- S. Du, F. Li, Q. Sun, N. Wang, M. Jia and J. Yu, Chem. Commun., 2016, 52, 3368–3371 RSC.
- Y. Cheneviere, F. Chieux, V. Caps and A. Tuel, J. Catal., 2010, 269, 161–168 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra09154g |
| This journal is © The Royal Society of Chemistry 2016 |