Unique quenching of fluorescent copper nanoclusters based on target-induced oxidation effect: a simple, label-free, highly sensitive and specific bleomycin assay†
Haiyin Li‡
,
Chuanfeng Wang‡,
Panpan Gai,
Ting Hou,
Lei Ge * and
Feng Li*
* and
Feng Li*
College of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University, Qingdao 266109, P. R. China. E-mail: lifeng@qust.edu.cn; lge@qau.edu.cn; Fax: +86 532 86080855; Tel: +86 532 86080855
First published on 9th August 2016
Abstract
In this contribution, a novel label-free fluorescence biosensor for bleomycin (BLM) detection was developed by combining the excellent fluorescence properties of copper nanoclusters (CuNCs) and the unique oxidation capability of a BLM–Fe2+ complex toward CuNCs. The CuNCs probe was prepared through in situ formation of CuNCs using single-stranded DNAs as the templates, endowing the probe with good water-dispersibility that is important for analyzing biological samples. After their recognition of BLM, the CuNCs were destroyed and the red fluorescence of the probe was quenched, thus realizing the detection of BLM. Such a fluorescence sensing strategy allows for highly sensitive BLM biosensing with a detection limit as low as 0.26 nM and minimal interference from complex mixtures. Compared to previously reported methods, the as-proposed assay does not need specific DNA sequences, complex designing or signal molecule labeling, and further avoids tedious experimental procedures, thus providing the strategy with additional advantages of simplicity and cost-effectiveness. Furthermore, our probe was also adopted for the detection of BLM in human serum samples and excellent performance was achieved, which makes the as-proposed strategy a promising candidate for highly sensitive and specific analysis of BLM in cancer treatment.
1. Introduction
Cancer has been one of the major threats to the lives of human beings for centuries.1,2 Nowadays, with the ever increasing environmental pollution and psychological pressure, the world's population is exposed to a high risk of global cancer incidence and mortality, which poses a severe threat to human health.3 So this spurs cancer therapy technology to become extremely important.4 Bleomycin (BLM), a typical antitumor drug, has been widely used in cancer therapies, considerably prolonging life due to its advantages of low myelosuppression and immunosuppression.5,6 However, excessive intake of BLM has been demonstrated to cause a wide range of diseases, such as pneumonitis and lung fibrosis, by accumulating in living organisms; nevertheless, insufficient dose of BLM may lead to poor curing effect.7–9 In this regard, it is urgent to develop simple, feasible and reliable methods for the detection of BLM in both pharmaceutical and clinical analysis, not only for monitoring the BLM dosage for curing cancers, but also for avoiding the potential harms caused by its toxicity.To date, a variety of BLM biosensors based on signal off–on strategy have been exploited. For instance, Ye et al. reported an electrochemical strategy based on ferrocene for BLM recognition, with a detection limit down to 100 pM.10 Ju et al. first reported a fluorescent probe based on Exo III aided DNA recycling amplification strategy for ultrasensitive detection of BLM.11 Our group also established a simple fluorescence turn-on sensing platform for trace BLM detection, with grapheme oxide and FAM-labeled single-stranded DNA (ssDNA) as the sensing elements.12 However, these probes have been designed based on the scission function of BLM specific to DNA sequence of 5′-GC/T-3′, and thus need complex DNA sequence designing and signal molecule labeling. Hence, it still remains a challenge to construct simple, facile and label-free sensing probes with high sensitivity and selectivity in response to BLM in both pharmaceutical analysis and clinical medicine research.
In this contribution, we reported a simple, cost-effective, highly sensitive and label-free fluorescent BLM biosensor based on the quenching of the strong fluorescence of CuNCs13–15 by BLM via the target-induced oxidation effect. The as-proposed probe was prepared through in situ formation of CuNCs using ssDNA as the templates. The adoption of DNA templates not only contributes to the formation of CuNCs, but also enhances the water-dispersibility against aggregation quenching the fluorescence of CuNCs. As a potential sensing probe, a specific chemical reaction between the probe and the target will give a unique signal change and provide us with versatile methods to determine a wide range of substances with excellent sensitivity and selectivity.16–22 The CuNCs are known to react with oxidizing agents to revert to the corresponding metal ion.23 In the presence of BLM, the CuNCs specifically react with BLM–Fe2+ complex and their fluorescence is quenched. Experimental results showed that the as-proposed CuNCs assay responded to BLM with high sensitivity and selectivity, and was successfully used to detect BLM in real samples.
2. Experimental
2.1 Materials
HPLC-purified poly(thymine), T40 with the sequence of 5′-T40-3′, was purchased from Sangon Biotech Company, Ltd. (Shanghai, China). 3-(N-Morpholino)propanesulfonic acid (MOPS), copper sulfate, sodium chloride, sodium ascorbate, and other salts were obtained from Dingguo Biotechnology Company, Ltd. (Beijing, China). Bleomycin sulfate (BLM) with the contents of A2 and B2 up to 91.6% was purchased from Melone Pharmaceutical Co. Ltd. (Dalian, China). The reagents were of analytical grade and used without further purification or treatment. Human serum samples were obtained from Qingdao Agriculture University Hospital. Ultra-pure water (resistivity > 18.2 MΩ cm@25 °C) obtained from a Milli-Q water purification system (Millipore Corp., Bedford, MA, USA) was used throughout the experiments.2.2 Synthesis of CuNCs
CuNCs were synthesized according to the reported method.24 Poly-T DNA, copper sulfate and sodium ascorbate were used as provided and diluted in 10 mM MOPS buffer solution (pH 7.6, containing 150 mM NaCl) to give the stock solutions. In a typical procedure, poly-T DNA solution and copper sulfate were mixed together. After pre-incubation for several minutes, sodium ascorbate was added into the above mixed solution to react for 5 minutes. The final volume of the solution was brought to 100 μL with MOPS buffer to keep DNA, copper sulfate and sodium ascorbate at the concentrations of 500 nM, 100 μM and 2 mM, respectively. The fluorescence signal was measured ranging from 520 nm to 660 nm.2.3 BLM sensing
The BLM samples were prepared by mixing BLM with Fe2+ ion in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. Then, the as-prepared probe solution and BLM sample with different concentrations were incubated at room temperature for selected time. The fluorescence signals of the mixtures were subsequently recorded ranging from 520 nm to 660 nm.
1 molar ratio. Then, the as-prepared probe solution and BLM sample with different concentrations were incubated at room temperature for selected time. The fluorescence signals of the mixtures were subsequently recorded ranging from 520 nm to 660 nm.
2.4 Apparatus and instrumentation
All fluorescence performances were detected on an F-4600 fluorescence spectrometer (Hitachi, Japan) equipped with a xenon lamp as the excitation source and a personal computer as the data processing unit. The slits for excitation and emission were set at 5.0 nm and 10.0 nm, respectively, with the excitation voltage of 700 V. The emission spectra from 520 nm to 660 nm were collected with the excitation wavelength set at 340 nm, and the FL intensity at 626 nm was utilized to evaluate the performance of the proposed BLM sensing strategy. UV-visible absorption spectra were recorded on an UV/Vis/NIR 2600 spectrometer (Shimadzu, Japan) using quartz cuvettes. Gel electrophoresis was carried out at 110 V for 40 min at room temperature, and stained for 20 min in a 1 × SYBR Green I solution. The resulting gel board was then illuminated with ultraviolet light and finally photographed by a Bio-Rad Gel Doc XR+ system (Hercules, CA, USA). Transmission electron microscopy (TEM) images were recorded on an HT7700 microscope (Hitachi, Japan) operated at 100 kV.3. Results and discussion
3.1 Principle of fluorescence BLM biosensing strategy
Given that poly-T DNA acts as the template for in situ formation of CuNCs, a ssDNA with 40 thymine was selected to construct the fluorescence probe for BLM detection. The as-proposed probe was expected to be oxidized by BLM–Fe2+ complex, resulting in the destruction of CuNCs, as well as the quenching of its strong fluorescence in the sensing process. Therefore, in the presence of BLM, the unique oxidizing complex BLM–Fe2+ was formed and oxidized the CuNCs, based on which, the luminescence of CuNCs was quenched. The decreased fluorescence intensity can be regarded as the signal for the sensing process. While in the absence of BLM, no oxidizing complex was generated, so no depression of the fluorescence intensity of CuNCs was observed. Therefore, highly sensitive and selective detection of BLM can be easily achieved by using the as-proposed CuNCs-based fluorescence sensing strategy (Scheme 1).The as-synthesized CuNCs were characterized by UV-Vis spectroscopy, fluorescence spectroscopy, and transmission electron microscopy (TEM). As clearly shown in Fig. 1A, as compared to DNA solution, a new UV-Vis absorption peak at 340 nm was observed for CuNCs aqueous solution. The CuNCs aqueous solution also exhibited a strong fluorescence peak at 626 nm (Fig. 1B), and the nanoaggregates with the diameters in the range of 2–5 nm were observed in TEM images (Fig. 1C), indicating the formation of copper nanoclusters. These results obviously demonstrated that CuNCs with strong fluorescence were successfully prepared through in situ reduction of Cu2+, and thus could be used as a sensing probe through fluorescence quenching effect.25,26
3.2 Feasibility study of fluorescent BLM assay
With the attempt for the fluorescence biosensing of BLM, CuNCs themselves should possess strong fluorescence in aqueous environment with excellent stability and become weakly emissive upon the addition of BLM. Considering these factors, the amount of sodium ascorbate, a reducing agent for CuNCs formation, was optimized to be 2.0 mM, as shown in Fig. S1A (ESI†). Under this experimental condition, the fluorescence biosensing of BLM was carried out. Upon the addition of BLM–Fe2+ complex into the sensing system, the red fluorescence was switched off as expected and the FL intensity at 626 nm decreased from 193.8 a.u. to 85.8 a.u. Such red fluorescence changes can also be distinguished by the naked-eye under UV light (Fig. 2A). Correspondingly, UV-Vis analysis revealed that CuNCs were destroyed as indicated by the disappearance of the absorption peak at 340 nm (Fig. 2B), which is consistent with the FL characterization. While only in the presence of either BLM or Fe2+, no obvious fluorescence decrease and UV-Vis spectra changes were observed, indicating the still existence of CuNCs (see Fig. S1B and C in ESI†). These results clearly demonstrated the feasibility of the as-proposed BLM assay based on fluorescence quenching strategy and the important role of BLM–Fe2+ complex for BLM detection.To investigate such a BLM induced fluorescence turn-off phenomenon, oxidation effect was considered as the main factor and was examined in detail. As is well known, in the presence of both Fe2+ and O2, BLM–Fe2+ complex was formed upon the addition of BLM; subsequently Fe2+ was oxidized to Fe3+, and oxygen was reduced to free radicals. The generated free radicals, possessing unique oxidation ability, could oxidize CuCNs to form Cu2+, and thus were the main factor to diminish the fluorescence signal output and to contribute to the destruction of CuNCs. In order to confirm that, H2O2, a typical oxidizing agent,27 was selected to substitute BLM–Fe2+ complex to characterize its oxidation effect toward CuNCs through the fluorescence quenching (Fig. 3A). As can be clearly seen, the fluorescence of CuNCs was quenched in the presence of H2O2, and with the H2O2 concentration increased to 50 μM, almost no fluorescence signal was detected. These fluorescence variations mainly derived from the destruction of CuNCs by H2O2, and were in accordance with that caused by BLM–Fe2+ complex. This successfully authenticated that, in the presence of O2, BLM–Fe2+ complex has strong oxidation ability and can destruct CuNCs to quench the fluorescence signal. To further study the mechanism of the as-proposed probe for BLM detection, ascorbic acid, a reducing agent,28 was adopted to pre-incubate with BLM–Fe2+ complex to diminish its oxidation effect toward CuNCs. After pre-incubation with ascorbic acid, BLM–Fe2+ complex could not quench the fluorescence of CuNCs and no obvious fluorescence changes were detected (Fig. 3B). These experimental results clearly demonstrated that the fluorescence decrease of CuNCs in the sensing process is due to the unique oxidation ability of BLM–Fe2+ complex in the presence of O2.
With in-depth investigation of the interaction between BLM–Fe2+ complex and CuNCs, the morphologies of CuNCs before and after the interaction with BLM–Fe2+ complex were characterized by TEM (Fig. 3C). As mentioned before, CuNCs possessed regular nanoaggregates with the diameters of 2–5 nm. After pre-incubation with BLM–Fe2+ complex, the CuNCs could not be observed, which is ascribed to the unique oxidation effect of BLM–Fe2+ toward CuNCs. This suggested that BLM–Fe2+ complex has strong oxidation ability and can destruct CuNCs, thus quenching their fluorescence and altering their UV-Vis absorbance property and morphologies. The gel electrophoresis analysis was also carried out to characterize the sensing process. Fig. S1D (ESI†) depicted that no obvious band difference was observed among DNA, CuNCs, and CuNCs after incubation with BLM–Fe2+, which further confirmed that the fluorescence decrease is mainly caused by the unique oxidation induced quenching effect from BLM–Fe2+ complex, not the changes of DNA strands.
3.3 Analytical performance of BLM assay
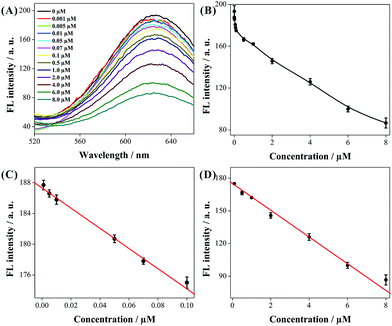
In order to obtain the best performance, some experimental conditions were optimized, including the reaction time between BLM–Fe2+ and CuNCs, and the selection of the optimal metal ion to form complex with BLM, and as shown in Fig. S2 (ESI†), 20 min was chosen as the optimum reaction time and Fe2+ as the optimal metal ion. Under the optimal experimental conditions, the dependence of the fluorescence emission of CuNCs on the concentration of BLM was investigated by incubation with BLM with the concentration ranging from 0 to 8 μM. As shown in Fig. 4A and B, the fluorescence intensity at 626 nm decreased gradually as the BLM concentration increased. Linear ranges for BLM detection were determined to be 0.001 to 0.1 μM (R2 = 0.9937, Fig. 4C) and 0.1 to 8.0 μM (R2 = 0.9948, Fig. 4D), respectively. The detection limit toward BLM was calculated to be 0.26 nM (based on 3S/N), much lower than those obtained by traditional methods,29,30 and comparable to that of the electrochemical assay and complicated fluorescence method.10–12 The low detection limit and the good linear correlation obtained here demonstrated that highly sensitive fluorescence detection of BLM can be successfully realized using the as-proposed approach.3.4 Selectivity of BLM assay
To study the selectivity of the probe, seven interfering molecules with different valences were examined as the alternatives of BLM for the interaction with CuNCs. As demonstrated in Fig. 5A, none of these interfering molecules could switch off the fluorescence of CuNCs under the same conditions, and only BLM could induce significant fluorescence quenching. Furthermore, to verify the ability of the probe to discriminate BLM in complex samples, BLM was analyzed in the mixture of the above seven compounds. Without any pretreatment, the mixed solution containing BLM emitted weak fluorescence, while fluorescence signal from the solution only consisting of seven compounds was significant. These results clearly indicated the high selectivity of the as-proposed CuNCs probe for BLM detection over other interfering species, which can facilitate the practical detection of BLM in complex biological samples.3.5 Application of BLM sensor in clinical samples
The applicability of the as-proposed assay for BLM detection in clinical samples was investigated. First, the fluorescence of CuNCs in human serum samples was studied. As shown in Fig. S3A (ESI†), the fluorescence spectra of CuNCs in MOPS buffer and in serum samples were almost the same, indicating no interference on the reaction specificity by various oxidizing and reducing agents in human serum. Then, the photostability of CuNCs in serum samples was also characterized. As demonstrated in Fig. S3B (ESI†), no evident fluorescence decline was observed during a one-hour period after CuCNs being added into the serum samples, with the FL intensity maintaining at about 193.8 a.u. Finally, recovery testing experiments were carried out by spiking BLM–Fe2+ samples into human serum. In the mixed solution, the volume proportion of serum was 50%. As clearly shown in Fig. S4 and Table S1 (ESI†), with the BLM concentration ranging from 10 nM to 60 nM, there was good agreement between the added and the measured values of BLM concentration, and the recoveries were found to be in the range of 96% to 108%, suggesting no severe interferences in these samples. Moreover, the relative standard deviations (RSD) of three repetitive measurements for each sample were all below 6%, demonstrating a relatively high reproducibility. These results indicate that the as-proposed CuNCs fluorescent sensor has good reliability and reproducibility, and can be successfully applied for the detection of BLM in clinical samples.4. Conclusions
In summary, based on the unique oxidation effect, we have, for the first time, developed a facile and label-free fluorescent sensing strategy for the highly sensitive and selective BLM determination. The sensing mechanism is mainly that the oxidation effect of BLM–Fe2+ complex toward CuNCs results in the quenching of the fluorescence of CuNCs. The as-proposed strategy realizes highly sensitive BLM detection with a detection limit down to 0.26 nM, and a highly specific recognition for BLM over other interfering molecules. More importantly, we demonstrated the real-world application of this assay by detecting BLM in human serum samples. This label-free fluorescence assay is simple, rapid, and cost effective, and thus holds great potential for the practical application in BLM detection in cancer diagnosis and treatment.Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 21575074, 21375072 and 21545005), the Basic Research Program of Qingdao (No. 14-2-4-102-jch), the Research Foundation for Distinguished Scholars of Qingdao Agricultural University (No. 663-1113334), and the Special Foundation for Taishan Scholar of Shandong Province.Notes and references
- L. Cheng, C. Wang, L. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869 CrossRef CAS PubMed.
- G. Lin, H. Zhang and L. Huang, Mol. Pharmaceutics, 2015, 12, 314 CrossRef CAS PubMed.
- L. Wu and X. Qu, Chem. Soc. Rev., 2015, 44, 2963 RSC.
- L. Feng, L. Wu and X. Qu, Adv. Mater., 2013, 25, 168 CrossRef CAS PubMed.
- J. C. Chapuis, R. M. Schmaltz, K. S. Tsosie, M. Belohlavek and S. M. Hecht, J. Am. Chem. Soc., 2009, 131, 2438 CrossRef CAS PubMed.
- U. Galm, W. W. Pienkowski, L. Wang, S. X. Huang, C. Unsin, M. Tao, J. M. Coughlin and B. J. Shen, J. Nat. Prod., 2011, 74, 526 CrossRef CAS PubMed.
- F. M. Kievit and M. Zhang, Adv. Mater., 2011, 23, H217 CrossRef CAS PubMed.
- S. Sleijfer, Z. Vujaskovic, P. C. Limburg, H. S. Koops and N. H. Mulder, Cancer, 1998, 82, 970 CrossRef CAS PubMed.
- Y. S. Choi, H. B. Kim, S. H. Kim, J. Choi and J. K. Park, Anal. Chem., 2009, 81, 3517 CrossRef CAS PubMed.
- B. Yin, D. Wu and B. Ye, Anal. Chem., 2010, 82, 8272 CrossRef CAS PubMed.
- F. Gao, J. Lei and H. Ju, Chem. Commun., 2013, 49, 7561 RSC.
- F. Li, Y. Feng, C. Zhao, P. Li and B. Tang, Chem. Commun., 2012, 48, 127 RSC.
- Y. Guo, F. Cao, X. Lei, L. Mang, S. Cheng and J. Song, Nanoscale, 2016, 8, 4852 RSC.
- L. Han, Y. Zhang, X. Chen, Y. Shu and J. Wang, J. Mater. Chem. B, 2016, 4, 105 RSC.
- N. Wang, B. Li, F. Qiao, J. Sun, H. Fan and S. Ai, J. Mater. Chem. B, 2015, 3, 7718 RSC.
- S. Gui, Y. Huang, F. Hu, Y. Jin, G. Zhang, L. Yan, D. Zhang and R. Zhao, Anal. Chem., 2015, 87, 1470 CrossRef CAS PubMed.
- X. Li, X. Zhang, Z. Chi, X. Chao, X. Zhou, Y. Zhang, S. Liu and J. Xu, Anal. Methods, 2012, 4, 3338 RSC.
- J. Tian, J. Zhou, Z. Shen, L. Ding, J. S. Yu and H. Ju, Chem. Sci., 2015, 6, 5969 RSC.
- W. Zhang, W. Liu, P. Li, F. Huang, H. Wang and B. Tang, Anal. Chem., 2015, 87, 9825 CrossRef CAS PubMed.
- M. H. Lee, J. H. Han, J. H. Lee, H. G. Choi, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2012, 134, 17314 CrossRef CAS PubMed.
- B. Tang, Y. Xing, P. Li, N. Zhang, F. Yu and G. Yang, J. Am. Chem. Soc., 2007, 129, 11666 CrossRef CAS PubMed.
- X. Liu, F. Wang, A. Niazov-Elkan, W. Guo and I. Willner, Nano Lett., 2013, 13, 309 CrossRef CAS PubMed.
- S. Zhou, Y. Li, F. Wang and C. Wang, RSC. Adv., 2016, 6, 38897 RSC.
- T. Ye, C. Li, C. Su, X. Ji, J. Zheng, P. Tinnefeld and Z. He, Chem. Commun., 2015, 51, 8644 RSC.
- H. Wang, H. Zhang, Y. Chen and Y. Liu, Biosens. Bioelectron., 2015, 74, 581 CrossRef CAS PubMed.
- H. Zhang, Z. Lin and X. Su, Talanta, 2015, 131, 59 CrossRef CAS PubMed.
- C. T. Nguyen and R. M. Kasi, Chem. Commun., 2015, 12174 RSC.
- S. M. Li, Y. S. Wang, S. T. Hsiao, W. H. Liao, C. W. Lin, S. Y. Yang, H. W. Tien, C. C. M. Ma and C. C. Hu, J. Mater. Chem. C, 2015, 3, 9444 RSC.
- R. P. Klett and J. P. Chovan, J. Chromatogr., 1985, 337, 182 CrossRef CAS PubMed.
- K. Fujiwara, M. Isobe, H. Saikusa, H. Nakamura, T. Kitagawa and S. Takahashi, Cancer Treat. Rep., 1983, 67, 363 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental sections and additional figures. See DOI: 10.1039/c6ra09054k |
| ‡ H. Y. Li and C. F. Wang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |