Preparation of a preferentially oriented SAPO-34 membrane by secondary growth under microwave irradiation†
Abstract
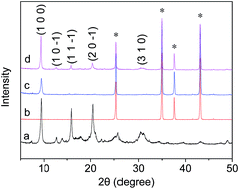
Piece-like SAPO-34 crystals were directly assembled into a highly oriented seed layer on the α-Al2O3 substrate by using spin-coating, and then a preferentially oriented SAPO-34 membrane with no large defects was prepared on an oriented SAPO-34 seed layer by secondary hydrothermal microwave growth. The synthesized membrane exhibited a high CO2 permeance of 1.57 × 10−6 s−1 Pa−1 at 302 K and 138 kPa pressure drop, with a CO2/CH4 separation selectivity of 109.

 Please wait while we load your content...
Please wait while we load your content...