1,10-Phenanthroline-2,9-dicarboxamides as ligands for separation and sensing of hazardous metals†
Abstract
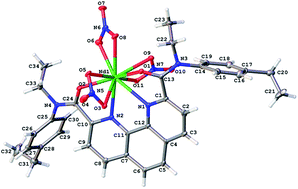
1,10-Phenanthroline-2,9-dicarboxamides of various structures were synthesized and studied as ligands for separation and sensing of d- and f-metals. It was found that the extraction ability of dialkyl-diaryl-diamide to lanthanides decreases from La to Lu and extraction of Am is close to light lanthanides (La–Pr). Tetraalkyl-diamide are not selective to lanthanides, instead exhibiting moderate selectivity in Am/Ln separation. The diamide complexes with lanthanides and d-elements were synthesized and characterized by XRD analysis. All diamides demonstrated good extraction ability to environmentally hazardous metals (cadmium, lead and copper). The synthesized compounds were also tested as ionophores in PVC-plasticized potentiometric sensor membranes. Such sensors displayed no perceptible response to lanthanides but exhibited high sensitivity towards copper, zinc, cadmium and lead. These compositions can be considered as promising cross-sensitive sensors for multisensor systems.


 Please wait while we load your content...
Please wait while we load your content...