A phosphotungstic acid-supported multifunctional organocatalyst containing 9-amino(9-deoxy)epi-cinchonidine and Brønsted acid and its application in asymmetric aldol reaction†
Abstract
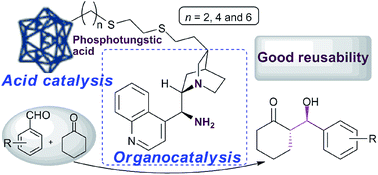
A novel type of supported multifunctional organocatalyst CDNH2(n)–HPW combined 9-amino(9-deoxy)epi-cinchonidine (CDNH2) with Brønsted acid in the backbone of phosphotungstic acid was developed via facile precipitation of sodium tungstate with organophosphonic acid. The aldol addition of cyclohexanone to o, m or p-substituted benzaldehydes bearing electron-withdrawing substituents (–X and –NO2) afforded good yields (71–96%) and excellent stereoselectivities (anti/syn = 94/6–98/2, 87–97% ee). It was found that the arm length (n) and acid capacity had significant effects on the cooperative catalysis of CDNH2 and Brøsted acid in the aldol reaction. After the completion of the aldol reaction, the catalysts could be readily recovered in quantitative yield by centrifugation and possessed good reusability with no significant loss of catalytic performance in the five consecutive asymmetric aldol reactions.

 Please wait while we load your content...
Please wait while we load your content...