Dehydrochlorination of 1,2-dichloroethane over Ba-modified Al2O3 catalysts†
Shuxing Baia,
Qiguang Dai*a,
Xinxin Chub and
Xingyi Wang*a
aKey Laboratory for Advanced Materials, Research Institute of Industrial Catalysis, East China University of Science and Technology, Shanghai 200237, PR China. E-mail: wangxy@ecust.edu.cn; daiqg@ecust.edu.cn; Fax: +86 21 64253372; Tel: +86 21 64253183
bKey Laboratory of Nuclear Radiation and Nuclear Energy Technology, Shanghai Institute of Applied Physics, Chinese Academy of Science, Shanghai 201800, PR China
First published on 19th May 2016
Abstract
Bimodal mesoporous alumina (Al2O3) was prepared using polyethyleneglycol (PEG 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and cetyl trimethyl ammonium bromide as a template. The incorporation of Ba with various loadings was carried out by incipient wetness. Characterization was performed by XRD, N2 sorption isotherms, and pyridine FTIR. Ba can be highly dispersed on Al2O3 covering the strong acid sites of Al2O3. In the catalytic dehydrochlorination of 1,2-dichloroethane (1,2-DCE), the Ba/Al2O3 catalysts present a high activity, of which Al2O3 is most active with 95% conversion at 325 °C, related to the more Lewis acidic Al3+ sites in a tetrahedral environment. 1,2-DCE adsorbs dissociatively on Lewis acid–base pair sites, forming chlorinated ethoxy species, which are supposed to be intermediate species for vinyl chloride (VC) production. At a temperature higher than 400 °C, the dehydrochlorination of VC occurs on the strong acid sites of Al2O3. Ba can promote greatly the selectivity for VC through a decrease in the strong acid sites. A high stable activity for dehydrochlorination and high selectivity for VC can be obtained over Ba/Al2O3 in the presence of oxygen.
000) and cetyl trimethyl ammonium bromide as a template. The incorporation of Ba with various loadings was carried out by incipient wetness. Characterization was performed by XRD, N2 sorption isotherms, and pyridine FTIR. Ba can be highly dispersed on Al2O3 covering the strong acid sites of Al2O3. In the catalytic dehydrochlorination of 1,2-dichloroethane (1,2-DCE), the Ba/Al2O3 catalysts present a high activity, of which Al2O3 is most active with 95% conversion at 325 °C, related to the more Lewis acidic Al3+ sites in a tetrahedral environment. 1,2-DCE adsorbs dissociatively on Lewis acid–base pair sites, forming chlorinated ethoxy species, which are supposed to be intermediate species for vinyl chloride (VC) production. At a temperature higher than 400 °C, the dehydrochlorination of VC occurs on the strong acid sites of Al2O3. Ba can promote greatly the selectivity for VC through a decrease in the strong acid sites. A high stable activity for dehydrochlorination and high selectivity for VC can be obtained over Ba/Al2O3 in the presence of oxygen.
1. Introduction
Vinyl chloride (VC) has been widely used in the production of popular homopolymeric and copolymeric plastic materials. The demand for VC as a basic commodity increases greatly with the application of vinyl plastic materials. The production of VC through chemical dehydrochlorination of l,2-dichloroethane (l,2-DCE) has been practised on a large scale, of which thermal dehydrochlorination of l,2-DCE at 450–500 °C is industrially a main route with 50% conversion and 98–99% selectivity for VC.1,2 Catalytic dehydrochlorination is highly effective in VC production. Generally, it was considered that the dehydrochlorination of chloroalkanes was promoted by solid base and acid catalysts. Shalygin reported l,2-DCE dehydrochlorination over a series of silicate catalysts, such as Al2O3/SiO2, Ga2O3/SiO2, ZrO2/SiO2, BeO/SiO2 and Y2O3/SiO2, which are acidic solid materials.1 There are a few reports on the interaction between chloroalkanes and Al2O3. Mochida studied the dehydrochlorination of several chloroalkanes (including 1,1,2-trichloroethane, 1,2-dichloropropane and 1,1,2-di-chloropropane) over Al2O3, base and acid catalysts under reductive reaction conditions.3 Dehydrochlorination reactions over dry Al2O3 were postulated to proceed through an E2-concerted mechanism, where the chlorine and hydrogen were eliminated almost simultaneously with the basic and acid sites of Al2O3. Feijen-Jeurissen4 reported the mechanism of catalytic decomposition of 1,2-DCE over γ-Al2O3, and proposed that the destruction of 1,2-DCE occurs through dehydrochlorination to VC. Catalytic cracking of DCE is also practised industrially over silicates, metal-promoted alumina, or zeolites, but the advantage balances the drawback of needing catalyst regeneration. The dehydrochlorination of chloride linear paraffins to linear olefins in the production of alkylbenzene sulfonate surfactants was performed catalytically in the presence of silica alumina or on metal packings of reactor columns acting as catalysts. In this case, no information is readily available in the open literature concerning catalyst stability.Recently, transitional alumina has been among the most used materials in any field of technologies, while details of its physicochemical properties are still a matter of discussion and investigation.5 The crystal structure of γ-Al2O3 is still a matter of controversy, having a defective non-stoichiometric spinel6,7 or other cubic or tetragonal structures with occupancy of non-spinel cationic sites.8–11 Digne et al.12,13 reported DFT results showing that the two main orientations (under practical operation conditions) of γ-Al2O3 facets were 〈100〉 (17%) and 〈110〉 (70%). The 〈100〉 facets were fully dehydrated at 600 K, leading to the formation of coordinatively unsaturated (penta-coordinate) Al3+. As reported, the acidity of γ-Al2O3 was related to coordination degree of the Al3+ species, of which the strongest Lewis acid sites were associated with very low coordination Al cations, such as tri- and tetra-coordinated Al3+. In the dehydration of ethanol, the most active sites were believed to be Lewis acidic Al3+ sites in a tetrahedral environment located on the edges and corners of the nanocrystals.14 In this paper, the surface structure of Al2O3 was modified with dropping Ba, and the effect of the corresponding acidity of Al2O3 on the activity, selectivity and stability in the catalytic dehydrochlorination of l,2-DCE was investigated. TPSR and in situ DRIFTS techniques were utilized to determine reaction intermediates and thus explore a possible reaction mechanism.
2. Experimental
2.1. Catalyst preparation
Bimodal mesoporous alumina (Al2O3) was synthesized using polyethyleneglycol (PEG 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and cetyl trimethyl ammonium bromide (CTAB) as a template. The typical procedure is described in ref. 15. CTAB (1.38 g), PEG (2.25 g) and aluminium isopropoxide (Al(O-i-Pr)3 5 g) were dissolved in ethanol aqueous solution (63% v/v) with vigorous stirring, and then ammonia (19 mL) as a precipitation agent was added and stirred for 30 min at 50 °C. The produced suspension was aged at 50 °C for 24 h under static conditions. The obtained filter was washed with ethanol and dried at 110 °C overnight. Dried samples were calcined for 3 h at 550 °C in air. Commercial Al2O3 (Al2O3-C) was referred to as a reference.
000) and cetyl trimethyl ammonium bromide (CTAB) as a template. The typical procedure is described in ref. 15. CTAB (1.38 g), PEG (2.25 g) and aluminium isopropoxide (Al(O-i-Pr)3 5 g) were dissolved in ethanol aqueous solution (63% v/v) with vigorous stirring, and then ammonia (19 mL) as a precipitation agent was added and stirred for 30 min at 50 °C. The produced suspension was aged at 50 °C for 24 h under static conditions. The obtained filter was washed with ethanol and dried at 110 °C overnight. Dried samples were calcined for 3 h at 550 °C in air. Commercial Al2O3 (Al2O3-C) was referred to as a reference.
A series of Ba/Al2O3 catalysts with various Ba loadings were prepared by conventional impregnation methods described elsewhere.9 The support Al2O3 (2 g) was impregnated with an aqueous solution (4 mL) (the content of barium nitrate was in a range of 0.0048–0.095 g mL−1 (0.05–10 wt%)) and then dried at 80 °C for 12 h and calcined in air at 550 °C for 3 h. The obtained catalysts were denoted as xBa/Al2O3 where x presents the content of Ba (wt%). Al2O3 used in this work is bimodal mesoporous Al2O3 unless stated otherwise.
2.2. Catalyst characterization
The powder X-ray diffraction (XRD) patterns of the samples were recorded on a Rigaku D/Max-rC powder diffractometer using Cu Kα radiation (40 kV and 100 mA). The diffractograms were recorded within the 2θ range of 10–80° with a 2θ step of 0.01° and a time step of 10 s. The nitrogen adsorption and desorption isotherms were measured at 77 K on a Micromeritics ASAP 2400 system operated in static measurement mode. Samples were outgassed at 350 °C for 4 h before the measurement. The specific surface area was calculated using the BET model. The actual Ba content was determined by inductively coupled plasma atomic emission spectrometry (ICP-AES) using a Varian 710 spectrometer. Samples were dissolved using an aqua regia-hydrogen peroxide system to form a homogeneous solution. The X-ray photoelectron spectroscopy (XPS) measurements were made on a VG ESCALAB MK II spectrometer using Mg Kα (1253.6 eV) radiation as the excitation source. Charging of the samples was corrected by setting the binding energy of adventitious carbon (C 1s) at 284.6 eV. The powder samples were pressed into self-supporting disks, and loaded into a sub-chamber, which was evacuated for 4 h prior to the measurements at 298 K. Temperature programmed surface reaction (TPSR) measurement was carried out under the same conditions as those in the catalytic activity tests. First, the feed containing 1000 ppm 1,2-DCE and Ar balance flowed continuously over the samples at 100 °C. After the adsorption–desorption reached an equilibrium, the samples were heated from 100 °C to a specified temperature at a heating rate of 10 °C min−1. The reactant and the products (such as DCE (m/z = 98), CO2 (44), CO or C2H4 (28), +CHO (29), Cl2 (70), HCl (36), C2H2 (26), C2H3Cl (62), C4 (41, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2+)) were analyzed on-line over a mass spectrometer apparatus (HIDEN, QIC-20).
CH–CH2+)) were analyzed on-line over a mass spectrometer apparatus (HIDEN, QIC-20).
2.3. Catalytic activity measurements
Catalytic dehydrochlorination was carried out at atmospheric pressure in a continuous flow microreactor (a quartz tube of 3 mm inner diameter). 75 mg catalyst (grain size, 40–60 mesh) was packed in the reactor bed. Before testing the activity and selectivity, the transport effects were investigated to ensure that the experimental results were not significantly influenced by interphase transportation. Calculation of the theoretical external transfer rate of reactants to the catalytic particles (at a typical temperature of 300 °C) based on estimated mass transfer coefficients gave a value magnitude three orders greater than the measured reaction rates, indicating that the process conditions were far from external diffusional limitations. The effects of external mass transfer resistances were experimentally evaluated by repeating a set of process conditions whilst employing a different linear velocity. The results of these experiments indicated that the conversion was not affected for a linear velocity higher than 7 cm s−1, within the experimental error. Likewise, estimates of the interphase temperature gradients showed fluid-solid differences of less than 1 °C. The possibility of internal pore diffusion was examined by measuring conversions at fixed conditions but varying the catalyst particle size. Results showed that the pore diffusional resistance was absent for particles less than 1 mm in diameter. Intraparticle mass transfer resistances were theoretically evaluated by computing effectiveness factors, which were calculated to be greater than 0.98, indicating that intraparticle mass transfer resistances were not significant. Finally, internal thermal gradients also proved to be negligible over the range of conditions evaluated in this study. The feed flow through the reactor was set at 40 mL min−1 (linear velocity of 9.4 cm s−1) and the gas hourly space velocity (GHSV) was maintained at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. The feed stream to the reactor was prepared by delivering liquid 1,2-DCE with a syringe pump into dry Ar and the injection position was electrically heated to ensure complete evaporation of the liquid reaction feeds. The temperature of the reactor was measured with a thermocouple located just at the bottom of the microreactor. The effluent gases were analyzed on-line at a given temperature using gas chromatography (GC9790, FULI) equipped with a Kromat-KB-5-30 m × 0.32 mm × 0.50 μm capillary column and a flame ionization detector (FID) for quantitative analysis of the organic chlorinated reactant. The catalytic activity was measured over the range 150–450 °C and the conversions were calculated by subtracting the outlet concentration from the inlet concentration of the reactant and dividing by the inlet concentration. These conversions were obtained at different temperatures under a steady state at each temperature. All the reactions were repeated three times to assure reproducibility. Furthermore, carbon balances could be as accurate as within 5%.
000 h−1. The feed stream to the reactor was prepared by delivering liquid 1,2-DCE with a syringe pump into dry Ar and the injection position was electrically heated to ensure complete evaporation of the liquid reaction feeds. The temperature of the reactor was measured with a thermocouple located just at the bottom of the microreactor. The effluent gases were analyzed on-line at a given temperature using gas chromatography (GC9790, FULI) equipped with a Kromat-KB-5-30 m × 0.32 mm × 0.50 μm capillary column and a flame ionization detector (FID) for quantitative analysis of the organic chlorinated reactant. The catalytic activity was measured over the range 150–450 °C and the conversions were calculated by subtracting the outlet concentration from the inlet concentration of the reactant and dividing by the inlet concentration. These conversions were obtained at different temperatures under a steady state at each temperature. All the reactions were repeated three times to assure reproducibility. Furthermore, carbon balances could be as accurate as within 5%.
2.4. In situ FTIR
In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) experiments were conducted on a Nicolet 6700 FTIR fitted with a liquid nitrogen-cooled mercury–cadmium–telluride detector (MCT). The DRIFTS cell (Harrick, HVC-DRP) fitted with ZnSe windows was used as the reaction chamber that allowed samples to be heated to 550 °C. All the spectra were obtained averaging 64 scans. Considering the instrumental optics and the strong framework adsorption of alumina, the useful spectral range was 4000–1100 cm−1. Prior to 1,2-DCE adsorption experiments, the samples were pre-treated in Ar at 550 °C for 2 h. Then the samples were cooled down to 50 °C in order to remove the contaminants. The spectra of the samples (20–30 mg) were recorded from 100 to 400 °C after 1,2-DCE adsorption following sweeping with Ar.3. Results and discussions
3.1. Catalyst characterization
 | ||
| Fig. 1 N2 sorption isotherms (a) and pore size distribution (b) of Al2O3, Ba/Al2O3 and Al2O3-C samples. | ||
| Sample | SBET (m2 g−1) | Vpore (cm3 g−1) | Dpore (nm) | Total aciditya (mmol per g cat) | Activity (°C) | ||
|---|---|---|---|---|---|---|---|
| Weak acid | Strong acid | T50 | T95 | ||||
| a Determination by NH3-TPD tests. | |||||||
| Al2O3 | 330 | 0.434 | 3/7 | 0.149 | 0.082 | 308 | 355 |
| 1Ba/Al2O3 | 301 | 0.366 | 3/6 | 0.162 | 0.100 | 315 | 360 |
| 2Ba/Al2O3 | 321 | 0.383 | 3/6 | 0.173 | 0.070 | 329 | 381 |
| 4Ba/Al2O3 | 334 | 0.390 | 2.7/5 | 0.108 | 0.060 | 338 | 404 |
| 10Ba/Al2O3 | 304 | 0.302 | 2.5/5 | 0.101 | 0.052 | 392 | 472 |
| Al2O3-C | 118 | 0.253 | 6 | 0.088 | 0.043 | 386 | 441 |
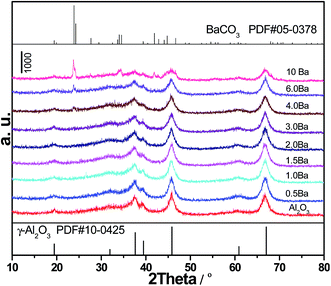
Fig. 3 shows HRTEM images of the synthesized Al2O3 and 4Ba/Al2O3 samples. With the presence of Ba, no evident change in morphology is observed. The size of the observed Al2O3 particles is in the range of 3–7 nm (white circles in Fig. 3a). The small Al2O3 particles were stacked together to form a large plane. The lattice spacing was measured to be 0.14 and 0.20 nm, in good agreement with those of the 〈440〉 and 〈400〉 crystal planes of the standard γ-Al2O3 sample (JCPDS 10-0425). The observation of electron diffraction rings in the selected area electron diffraction (SAED) patterns (inset of Fig. 3a) suggests the formation of a polycrystalline structure. The images also show that the catalysts are homogeneous with the absence of crystalline Ba oxide or BaCO3 phases. To further characterize the distribution of Ba in 4Ba/Al2O3, STEM mapping of Ba, Al and O was conducted. As shown in Fig. S1,† Ba species were uniformly and highly dispersed on the Al2O3 surface. This result shows the strong interaction between Ba and Al2O3, as reported elsewhere.9,16,17 A low BaO coverage of 2 wt% on γ-Al2O3 monomeric BaO units was present almost exclusively and these molecularly dispersed BaO units were concentrated on the (100) faces of the alumina crystallites.9 Density functional theory calculations predicted that the energetically most favorable BaO monomer and dimer units anchor to the penta-coordinate Al3+ sites on the (100) faces of γ-Al2O3 in such geometries that maximize their interactions with the support surface.16 In our case, Al2O3 exposed mainly 〈400〉, which is really different from the above results.9,16 The BaO monomer and dimer units should be formed because the aggregation of BaO can not be observed on 4Ba/Al2O3 where the Ba species is highly dispersed on Al2O3 (confirmed by HRTEM). Additionally, the crystalline particles of BaCO3 are not detected by HRTEM, indicating that XRD diffraction from BaCO3 may be due to a separate phase from Al2O3 produced during calcination.
3.2. Dehydrochlorination
With incorporation of Ba, the conversion curve shifts to a high temperature. T95% is proportional to the Ba loading (Table 1) and increases from 350 °C for Al2O3 to 471 °C for 10Ba/Al2O3, indicating that the activity is inhibited by Ba to some extent. A similar product distribution over catalysts containing Ba is available. However, the aldehyde distribution becomes broader and higher with an increase in the Ba loading and the selectivity for aldehyde over 4Ba/Al2O3 reaches 60% at 225 °C. In all cases, the higher the 1,2-DCE conversion is, the higher the VC selectivity is; and the lower the DCE conversion is, the higher the aldehyde selectivity is. At complete conversion, aldehyde is not formed at all, suggesting that the 1,2-DCE partial pressure available is a key factor favoring aldehyde (at low conversion, with more aldehyde available) or VC (at high conversion). In fact, the dependence of the aldehyde formation is expected to have a higher reaction order with respect to 1,2-DCE than VC synthesis. On the other hand, the presence of Ba can produce additional oxygen species as strong basic sites, and so may be favorable for the formation of aldehyde through nucleophilic attack (see later). Moreover, the formation of ethyne during increasing the temperature decreases gradually with Ba addition. At 425 °C, the selectivity for ethyne decreases from 60% over Al2O3 to 5% over 4Ba/Al2O3. The elimination of HCl from chloroalkanes is promoted by solid base and acid catalysts. Generally, the reactivity of the chlorinated ethylenes decreases with the increasing chlorine content in the molecule. The rate determining step probably does not involve breaking the C–Cl or C–H bonds. Bond et al.25 suggested that over a Pt/Al2O3 catalyst, the rate determining step is the removal of a chlorine atom. Chintawar et al. extensively studied the oxidation of chlorinated ethylenes.26 They found a strong correlation between the adsorption capacity of the molecule on the catalyst and the reactivity of the molecule. The elimination reactions of the chlorocompounds over Al2O3 in a dry feed were postulated to proceed through an E2-concerted mechanism where the chlorine and proton were eliminated almost simultaneously by the acidic and basic sites of Al2O3. If the acidity of the proton was weak enough, such as that in the case of VC, the removal of the Cl atom on the strong acid sites was critical. At that time, the elimination may proceed via an E1-concerted mechanism. For Ba/Al2O3 catalysts, the decrease in strong acid sites is really not favorable for the activation of VC, even though the basicity of the Ba/Al2O3 catalysts is strong. As expected, Al2O3-C with less strong acidity shows a poor selectivity for ethyne at high temperature. Al2O3-C with less strong basic sites (basic oxygen species such as BaO possesses) presents a lower selectivity for aldehyde (Fig. 7).
 | ||
| Fig. 7 The distribution of products obtained in the reactions under the same conditions as those described in Fig. 6. | ||
In order to investigate the effect of acidity on catalysts on the kinetics of 1,2-DCE dehydrochlorination, TOF based on the mole number of converted 1,2-DCE molecules per second per mole of acidic site on the surface of catalysts can be used for comparing the activity difference among the acidic sites of Al2O3 and Ba/Al2O3. Fig. 6 (inset) shows the TOF at 300 °C as a function of Ba loading. It can be seen that the TOF decreases almost linearly with the Ba loading. A few studies have been published on the interaction between chloroethanes and Al2O3. A significant difference in TOF indicates that the rate determining step probably does not involve breaking C–Cl bonds. The removal of Cl species from the surface may be a slow step (Fig. 8).27
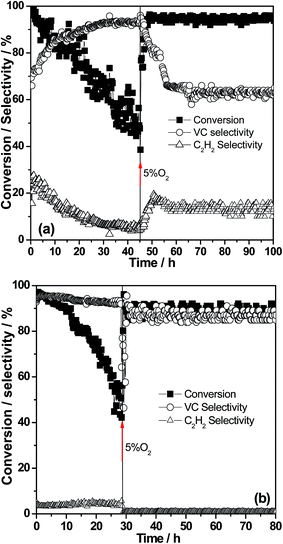 | ||
Fig. 9 The stability on the feed streams at 400 °C of the Al2O3 (a) and 4Ba/Al2O3 (b) catalysts. Reaction gas: 1000 ppm 1,2-DCE and Ar balance; SV = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 SV = 30 000 SV = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. 000 mL g−1 h−1. | ||
In the dry and oxygen-free feed, the selectivity of VC increases gradually with the deactivation of Al2O3 and reaches 96% at a conversion of 50%, due to the decrease in strong acidic sites through carbon deposition. However, with the removal of carbon from the strong acidic sites in the feed containing oxygen, the dehydrochlorination of VC becomes significant, and the selectivity for VC finally decreases to about 65%. This phenomenon indicates that the strong acidic sites on which black carbon deposits are responsible for the second dehydrochlorination. It is interesting to find that for 4Ba/Al2O3, the selectivity for VC maintains 90% during the stability test (at least 80 h) and almost a zero amount of ethyne was detected. 4Ba/Al2O3 in particular was tested in the feed containing 5% oxygen for another 80 h at 400 °C with a conversion as high as 90–92% and the selectivity for VC was as high as 88–90% (ESI†). This result is really different from those obtained previously, in which catalytic cracking at 300–400 °C on pumice (SiO2, Al2O3, alkalis) or on charcoal, impregnated with BaCl2 or ZnCl2 has not found more widespread application due to the limited life of the catalysts of about 10 h and VC can be further oxychlorinated to ethyl trichloride and further transformed in the presence of oxygen.31 To the best of our knowledge, the catalysts used for dehydrochlorination of chlorinated linear paraffins were acidic catalysts, such as silicates and zeolites.1,3,32,33 Similar results were found in the dehydrochlorination of 1,2-dichloropropane over silica-alumina catalysts where 20% selectivity for allyl chloride and 10 h stability were available.34 The theoretical and experimental studies showed that the addition of Ba species can cover strong acidic sites of Al2O3.16 It provided an opportunity to develop a new pathway of 1,2-DCE dehydrochlorination over catalysts with strong basicity and weak acidity. The synergy of a strong base and weak acid is critical to ensure a good performance of 4Ba/Al2O3. The carbon balance can reach 95% or higher. CO can be detected in effluent due to the oxidation of 1,2-DCE. Inorganic Cl species leave from the reactor as HCl but not as Cl2. In fact, a significant Deacon reaction (HCl + O2 → Cl2 + H2O) occurs at 700 °C or higher over Al2O3.35
In order to understand the high selectivity for VC in the presence of oxygen, the oxidation of VC over Al2O3 and 4Ba/Al2O3 was conducted. The activity of Al2O3 and 4Ba/Al2O3 for VC oxidation is poor, and the conversion reaches 56% and 20% up to 350 °C (Fig. S6†). Moreover, the addition of 1,2-DCE can retard the conversion of VC, indicating that 1,2-DCE is more favorable for adsorption on active sites. High selectivity for VC and a stable activity over 4Ba/Al2O3 in the presence of oxygen results from its high activity for dehydrochlorination of 1,2-DCE and low activity for the second dehydrochlorination and VC oxidation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–90
000–90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. The results (Fig. 10 and S7†) show that the increase in space velocities does not decrease the rate (based on the mole number of 1,2-DCE converted per second per square meter), which is not as expected. The highest reaction rate was obtained at 60
000 mL g−1 h−1. The results (Fig. 10 and S7†) show that the increase in space velocities does not decrease the rate (based on the mole number of 1,2-DCE converted per second per square meter), which is not as expected. The highest reaction rate was obtained at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1 and the rates at 30
000 mL g−1 h−1 and the rates at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 90
000 and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1 are almost equal. These anomalous data arevassociated with the promotion of the removal of Cl species produced during the reaction by a higher linear rate of feed in the reaction bed, implying that at a lower feed rate, HCl desorbed from the surface can go back to the surface. Busca observed this anomalous phenomenon in the dehydration of ethanol over Al2O3 containing more chlorine impurities.14
000 mL g−1 h−1 are almost equal. These anomalous data arevassociated with the promotion of the removal of Cl species produced during the reaction by a higher linear rate of feed in the reaction bed, implying that at a lower feed rate, HCl desorbed from the surface can go back to the surface. Busca observed this anomalous phenomenon in the dehydration of ethanol over Al2O3 containing more chlorine impurities.14
3.3. In situ FTIR spectra
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOH) in the gas phase show a strong absorption band between 1644 and 1648 cm−1, which is accompanied by two bands at 1409–1412 and 1300–1326 cm−1.41,42 The IR spectrum of adsorbed catechol on a TiO2 colloid also gives a similar band at 1620 cm−1.43 Their common characteristic is an enolic structure. On the other hand, a band at 1605 cm−1 grows substantially with temperature on stream. Dreoni et al.44 assigned a strong band observed at 1598 cm−1 during adsorption of cyclohexanone on silica at 200 °C to a C
CHOH) in the gas phase show a strong absorption band between 1644 and 1648 cm−1, which is accompanied by two bands at 1409–1412 and 1300–1326 cm−1.41,42 The IR spectrum of adsorbed catechol on a TiO2 colloid also gives a similar band at 1620 cm−1.43 Their common characteristic is an enolic structure. On the other hand, a band at 1605 cm−1 grows substantially with temperature on stream. Dreoni et al.44 assigned a strong band observed at 1598 cm−1 during adsorption of cyclohexanone on silica at 200 °C to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration. Furthermore, these authors also assigned bands at 3075 and 3045 cm−1 to unsaturated
C stretching vibration. Furthermore, these authors also assigned bands at 3075 and 3045 cm−1 to unsaturated ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– bond stretching. Indeed, a similar band at 3040 cm−1 is also observed in our case during the adsorption of 1,2-DCE and can be assigned to such a vibration mode. Additional bands in the same region at 2939 and 2867 cm−1 correspond to C
CH– bond stretching. Indeed, a similar band at 3040 cm−1 is also observed in our case during the adsorption of 1,2-DCE and can be assigned to such a vibration mode. Additional bands in the same region at 2939 and 2867 cm−1 correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and –CH– bonds and are also present in the spectra of adsorbed 1,2-DCE. The presence of C
C and –CH– bonds and are also present in the spectra of adsorbed 1,2-DCE. The presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and –CH– bonds further verifies the formation of the enolic form.44 The bands at 1386, 1268 and 1605 cm−1 (assigned to δCH2, ρCH and γC
C and –CH– bonds further verifies the formation of the enolic form.44 The bands at 1386, 1268 and 1605 cm−1 (assigned to δCH2, ρCH and γC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of VC) become significant up to 250 °C, where the conversion to VC in a parallel kinetic reaction is significant. At the same time, new bands appear at 1680 and 1730 cm−1, ascribed to the –C
C of VC) become significant up to 250 °C, where the conversion to VC in a parallel kinetic reaction is significant. At the same time, new bands appear at 1680 and 1730 cm−1, ascribed to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. Generally, adsorbed acetaldehyde corresponded to coordination to a Lewis acid, R–CH
O group. Generally, adsorbed acetaldehyde corresponded to coordination to a Lewis acid, R–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–Al3+.4 In fact, –CHO was detected in TPSR as m/z = 29, and in a parallel kinetic reaction, a significant amount of aldehyde in the effluent can be observed. Chintawar et al. observed for the adsorption of vinyl chloride on chromium-exchanged zeolite Y a band at 1678 cm−1 and assigned this band to an adsorbed aldehyde or ketone.40 Zhou observed the formation of aldehyde during the decomposition of 1,2-DCE at 260–360 °C.45 VC can readily be protonated in the presence of acid catalysts, like AlCl3, in the presence of OH surface species into a stable reactive carbonium ion which then was attacked by a nucleophilic oxygen species (basic site of alumina or adsorbed water), leading to the CH3–CHCl–O species, which would readily decompose to form acetaldehyde and leave a chloride ion on the surface. Moreover, new bands appear at 1338–1356 and 1565 cm−1 (assigned to carbonate bidentate), 1268, 1425 and 1538 cm−1 (asymmetric stretching vibration of carboxylates of the acetate type),40,46–48 and at 1470 and 1356 cm−1 (monodentate bonded carbonates)49 at 250 °C or higher, probably due to the presence of surface oxygen species, such as basic hydroxyl groups.
O–Al3+.4 In fact, –CHO was detected in TPSR as m/z = 29, and in a parallel kinetic reaction, a significant amount of aldehyde in the effluent can be observed. Chintawar et al. observed for the adsorption of vinyl chloride on chromium-exchanged zeolite Y a band at 1678 cm−1 and assigned this band to an adsorbed aldehyde or ketone.40 Zhou observed the formation of aldehyde during the decomposition of 1,2-DCE at 260–360 °C.45 VC can readily be protonated in the presence of acid catalysts, like AlCl3, in the presence of OH surface species into a stable reactive carbonium ion which then was attacked by a nucleophilic oxygen species (basic site of alumina or adsorbed water), leading to the CH3–CHCl–O species, which would readily decompose to form acetaldehyde and leave a chloride ion on the surface. Moreover, new bands appear at 1338–1356 and 1565 cm−1 (assigned to carbonate bidentate), 1268, 1425 and 1538 cm−1 (asymmetric stretching vibration of carboxylates of the acetate type),40,46–48 and at 1470 and 1356 cm−1 (monodentate bonded carbonates)49 at 250 °C or higher, probably due to the presence of surface oxygen species, such as basic hydroxyl groups.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–Al3+. The same phenomenon is observed on Al2O3-C with less strong acid sites (Fig. S8†), where the carbonate bidentate, carboxylates of the acetate type and monodentate bonded carbonates can not be observed within the experimental temperature. These results imply that the oxidation involving surface oxygen species on Al2O3-C is weak, probably related to the lack of strong basic sites or strong acid sites.
O–Al3+. The same phenomenon is observed on Al2O3-C with less strong acid sites (Fig. S8†), where the carbonate bidentate, carboxylates of the acetate type and monodentate bonded carbonates can not be observed within the experimental temperature. These results imply that the oxidation involving surface oxygen species on Al2O3-C is weak, probably related to the lack of strong basic sites or strong acid sites.
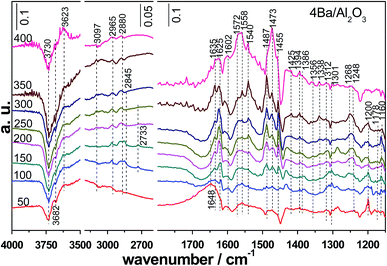 | ||
| Fig. 12 In situ FTIR spectra in the 1100–4000 cm−1 region for 4Ba/Al2O3 in a 1000 ppm 1,2-DCB/Ar stream from 50 to 400 °C after treatment in Ar at 550 °C. | ||
Based on these experiments it was postulated that the first step in the interaction of 1,2-DCE with Lewis acid sites occurs and the adsorbed 1,2-DCE can undergo nucleophilic attack by oxygen to form a chlorinated ethoxy intermediate through the removal of a chlorine atom. The formation of VC occurs through transfer of a proton and rearrangement of chlorinated ethoxy. The rearrangement of the ethoxy intermediate was considered to be an indispensable step in the dehydration of ethanol on pure Al2O3.14 At the same time, VC adsorbed on two sites, a pair of acidic-basic sites can be converted into carbonyl species which may or may not contain chlorine, and be stabilized by resonance (an enolic structure). And with the destruction of VC, the formation of aldehyde was proposed. A following oxygen attack on the carbonyl compound results in the formation of carboxylate and carbonate species.40 On the other hand, on strong acidic sites, the chlorine atom of VC can be abstracted with the transfer of a proton, and ethyne is formed. In order to obtain deep insights into this reaction at a molecular level, a mechanism over Al2O3 consisting of three elementary steps is drawn (Fig. 13): (1) formation of the 1,2-DCE adsorption complex during the interaction of 1,2-DCE with Lewis acid sites; (2) chlorine abstraction by nucleophilic oxygen (surface hydroxyl groups) to form the chlorinated ethoxy intermediate; and (3) dehydroxylation through rearrangement to form VC. Other side reactions include: (1) attack of basic oxygen species to VC; (2) formation of oxygenate species, such as aldehydes, carbonate bidentates, monodentates and partially oxidized surface species such as enolic species, acetates, and carboxylates of the acetate type; and (3) the formation of ethyne through the interaction of VC with strong Lewis acid sites, such as tetrahedral Al3+ ions at high temperature.
4. Conclusions
Bimodal mesoporous Al2O3 was prepared using polyethyleneglycol (PEG 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and cetyl trimethyl ammonium bromide as a template. Ba/Al2O3 catalysts with a Ba loading of 1–10 wt% obtained by incipient wetness were characterized by XRD, BET and porosity measurements, and Py-FTIR, and used in the catalytic dehydrochlorination of 1,2-DCE. The results showed that the Ba species was highly dispersed on Al2O3 probably as monomeric or dimeric BaO. The number of surface tetrahedral Al3+ ions decreases with the Ba loading, which corresponds to the decrease in strong acid sites. In the catalytic dehydrochlorination of 1,2-DCE, the Ba/Al2O3 catalysts present a high activity, of which Al2O3 is most active with 95% conversion at 325 °C. The products are composed of VC, aldehyde, ethyne and butene. The formation of aldehyde occurs at low 1,2-DCE conversion (at low temperature) and butane appears at a higher temperature with a low selectivity (below 1%). For Al2O3, the selectivity for VC reaches 95% at 350 °C, however, at a higher temperature, the selectivity decreases quickly and is only 40% at 425 °C, which is related to a significant formation of ethyne. The addition of Ba really promotes the selectivity for VC at high temperature through the decrease in strong acidic sites which are favorable for the second dehydrochlorination. Al2O3 and 4Ba/Al2O3 deactivate heavily on the stream within 20 h at 400 °C, due to the deposition of carbon and chlorine species. The presence of oxygen in a small amount can effectively promote the stability, in which 90% conversion and 90% selectivity for VC on 4Ba/Al2O3 is available. In situ FTIR showed that the first step in the interaction of 1,2-DCE with Lewis acid sites occurs and the adsorbed 1,2-DCE can undergo nucleophilic attack by oxygen to form a chlorinated ethoxy intermediate through the removal of a chlorine atom. The formation of VC occurs through transfer of a proton and rearrangement of chlorinated ethoxy. Different types of partially oxidized products, including the enolic form of the aldehyde-type intermediate, were observed because of the attack to VC by hydroxyl or basic oxygen species existing on the surface of the Al2O3 or 4Ba/Al2O3 catalysts.
000) and cetyl trimethyl ammonium bromide as a template. Ba/Al2O3 catalysts with a Ba loading of 1–10 wt% obtained by incipient wetness were characterized by XRD, BET and porosity measurements, and Py-FTIR, and used in the catalytic dehydrochlorination of 1,2-DCE. The results showed that the Ba species was highly dispersed on Al2O3 probably as monomeric or dimeric BaO. The number of surface tetrahedral Al3+ ions decreases with the Ba loading, which corresponds to the decrease in strong acid sites. In the catalytic dehydrochlorination of 1,2-DCE, the Ba/Al2O3 catalysts present a high activity, of which Al2O3 is most active with 95% conversion at 325 °C. The products are composed of VC, aldehyde, ethyne and butene. The formation of aldehyde occurs at low 1,2-DCE conversion (at low temperature) and butane appears at a higher temperature with a low selectivity (below 1%). For Al2O3, the selectivity for VC reaches 95% at 350 °C, however, at a higher temperature, the selectivity decreases quickly and is only 40% at 425 °C, which is related to a significant formation of ethyne. The addition of Ba really promotes the selectivity for VC at high temperature through the decrease in strong acidic sites which are favorable for the second dehydrochlorination. Al2O3 and 4Ba/Al2O3 deactivate heavily on the stream within 20 h at 400 °C, due to the deposition of carbon and chlorine species. The presence of oxygen in a small amount can effectively promote the stability, in which 90% conversion and 90% selectivity for VC on 4Ba/Al2O3 is available. In situ FTIR showed that the first step in the interaction of 1,2-DCE with Lewis acid sites occurs and the adsorbed 1,2-DCE can undergo nucleophilic attack by oxygen to form a chlorinated ethoxy intermediate through the removal of a chlorine atom. The formation of VC occurs through transfer of a proton and rearrangement of chlorinated ethoxy. Different types of partially oxidized products, including the enolic form of the aldehyde-type intermediate, were observed because of the attack to VC by hydroxyl or basic oxygen species existing on the surface of the Al2O3 or 4Ba/Al2O3 catalysts.
Acknowledgements
This research was supported by Development Program for National Natural Science Foundation of China (No. 21277047, 21477036 and 21307033).References
- A. S. Shalygin, L. M. Koval, L. V. Malysheva, N. S. Kotsarenko and E. A. Paukshtis, Chem. Sustainable Dev., 2009, 17, 417–422 Search PubMed.
- A. Lakshmanan, W. C. Rooney and L. T. Biegler, Comput. Chem. Eng., 1999, 23, 479–495 CrossRef CAS.
- I. Mochida, A. Uchino, H. Fujitsu and K. Takeshita, J. Catal., 1978, 51, 72–79 CrossRef CAS.
- M. M. R. Feijen-Jeurissen, J. J. Jorna, B. E. Nieuwenhuys, G. Sinquin, C. Petit and J. P. Hindermann, Catal. Today, 1999, 54, 65–79 CrossRef CAS.
- G. Busca, Catal. Today, 2014, 226, 2–13 CrossRef CAS.
- B. C. Lippens and J. H. de Boer, Acta Crystallogr., 1964, 17, 1312–1321 CrossRef CAS.
- A. R. Ferreira, M. J. F. Martins, E. Konstantinova, R. B. Capaz, W. F. Souza, S. S. X. Chiaro and A. A. Laitão, J. Solid State Chem., 2011, 184, 1105–1111 CrossRef CAS.
- R. S. Zhou and R. L. Snyder, Acta Crystallogr., Sect. B: Struct. Sci, 1991, 47, 617–630 CrossRef.
- J. H. Kwak, J. Z. Hu, D. H. Kim, J. Szanyi and C. H. F. Peden, J. Catal., 2007, 251, 189–194 CrossRef CAS.
- G. Paglia, A. L. Rohl, C. E. Buckley and J. D. Gale, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 224115–224116 CrossRef.
- L. Smrcok, V. Langer and J. Krestan, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2006, 62, 83–84 Search PubMed.
- M. Digne, P. Sautet, P. Raybaud, P. Euzen and H. Toulhoat, J. Catal., 2004, 226, 54–68 CrossRef CAS.
- M. Digne, P. Sautet, P. Raybaud, P. Euzen and H. Toulhoat, J. Catal., 2002, 211, 1–5 CrossRef CAS.
- T. K. Phung, A. Lagazzo, M. Á. R. Crespo, V. S. Escribano and G. Busca, J. Catal., 2014, 311, 102–113 CrossRef CAS.
- P. Bai, P. P. Wu and Z. F. Yan, Microporous Mesoporous Mater., 2009, 118, 288–295 CrossRef CAS.
- H. Kwak, D. Mei, C. W. Yi, D. H. Kim, C. H. F. Peden, L. F. Allard and J. Szanyi, J. Catal., 2009, 261, 17–22 CrossRef.
- J. H. Kwak, J. Z. Hu, D. Mei, C. W. Yi, D. H. Kim, C. H. F. Peden, L. F. Allard and J. Szanyi, Science, 2009, 325, 1670–1673 CrossRef CAS PubMed.
- H. Knözinger and P. Ratnasamy, Catal. Rev.: Sci. Eng., 1978, 17, 31–70 Search PubMed.
- C. Morterra and G. Magnacca, Catal. Today, 1996, 27, 497–532 CrossRef CAS.
- X. Liu and R. E. Truitt, J. Am. Chem. Soc., 1997, 119, 9856–9860 CrossRef CAS.
- D. T. Lundie, A. R. McInroy, R. Marshall, J. M. Winfield, P. Jones, C. C. Dudman, S. F. Parker, C. Mitchell and D. Lennon, J. Phys. Chem. B, 2005, 109, 11592–11601 CrossRef CAS PubMed.
- S. Bordiga, C. Lamberti, F. Bonino, A. Travertd and F. T. Starzyk, Chem. Soc. Rev., 2015, 44, 7262–7341 RSC.
- G. Busca, Chem. Rev., 2010, 110, 2217–2249 CrossRef CAS PubMed.
- A. E. Kulikova and E. N. Zilberman, Russ. Chem. Rev., 1971, 40, 256–271 CrossRef.
- G. C. Bond and N. Sadeghi, J. Appl. Chem. Biotechnol., 1975, 25, 241–248 CAS.
- P. S. Chintawar and H. L. Greene, Appl. Catal., B, 1997, 13, 81–92 CrossRef CAS.
- L. Ran, Z. Qin, Z. Y. Wang, X. Y. Wang and Q. G. Dai, Catal. Commun., 2013, 37, 5–8 CrossRef CAS.
- G. Clet, J. M. Goupil and D. Cornet, Bull. Soc. Chim. Fr., 1997, 134, 223–233 CAS.
- A. G. Goble and P. A. Lawrance, in Proc. 3rd Int. Cong. Catal., North Holland, Amsterdam, 1964, vol. I, p. 320 Search PubMed.
- N. B. Muddada, U. Olsbye, T. Fuglerud, S. Vidotto, A. Marsella, S. Bordiga, D. Gianolio, G. Leofanti and C. Lamberti, J. Catal., 2011, 284, 236–246 CrossRef CAS.
- G. Leofanti, A. Marsella, B. Cremaschi, M. Garilli, A. Zecchina, G. Spoto, S. Bordiga, P. Fisicaro, G. Berlier, C. Prestipino, G. Casali and C. Lamberti, J. Catal., 2001, 202, 279–295 CrossRef CAS.
- K. Weissermel and H. J. Arpe, Industrial Organic Chemistry, VCH, Weinheim, Germany, 3rd edn, 1997, p. 219 Search PubMed.
- E. L. Dreher, Ullmann’s Encyclopedia of Industrial Chemistry, VCH, Weinheim, Germany, 1986, vol. A6, p. 289 Search PubMed.
- C. Pistarino, E. Finocchio, M. A. Larrubia, B. Serra, S. Braggio and G. Busca, Ind. Eng. Chem. Res., 2001, 40, 3262–3269 CrossRef CAS.
- M. W. M. Hisham and S. W. Benson, J. Phys. Chem., 1995, 99, 6194–6198 CrossRef CAS.
- D. Carmello, E. Finocchio, A. Marsella, B. Cremaschi, G. Leofanti, M. Padovan and G. Busca, J. Catal., 2000, 191, 354–363 CrossRef CAS.
- B. H. Aristizábal, C. M. de Correa, A. I. Serykh, C. E. Hetrick and M. D. Amiridis, Microporous Mesoporous Mater., 2008, 112, 432–440 CrossRef.
- H. Vigué, P. Quintard, T. Merle-Méjean and V. Lorenzelli, J. Eur. Ceram. Soc., 1998, 18, 305–309 CrossRef.
- A. Starczewska, R. Wrzalik, M. Nowak, P. Szperlich, Ł. Bober, J. Szala, D. Stroz and D. Czechowicz, Infrared Phys. Technol., 2008, 51, 307–315 CAS.
- P. S. Chintawar and H. L. Greene, J. Catal., 1997, 165, 12–21 CrossRef CAS.
- M. Mikami, I. Nakagawa and T. Shimanouchi, Spectrochim. Acta, 1967, 23A, 1037–1053 CrossRef.
- M. Rodler, C. E. Blom and A. Bauder, J. Am. Chem. Soc., 1984, 106, 4029–4035 CrossRef CAS.
- T. Rajh, L. X. Chen, K. Lukas, T. Liu, M. C. Thurnauer and D. M. Tiede, J. Phys. Chem. B, 2002, 106, 10543–10552 CrossRef CAS.
- D. P. Dreoni, D. Pinelli, F. Trifiro, G. Busca and V. Lorenzelli, J. Mol. Catal., 1992, 71, 111–127 CrossRef CAS.
- Q. Q. Huang, Z. H. Meng and R. X. Zhou, Appl. Catal., B, 2012, 115–116, 179–189 CrossRef CAS.
- S. Krishnamoorthy, J. A. Rivas and M. D. Amiridis, J. Catal., 2000, 193, 264–272 CrossRef CAS.
- V. E. Suprunov and A. A. Ivanov, React. Kinet. Catal. Lett., 1987, 33, 75–80 CrossRef CAS.
- V. S. Escribano, G. Busca and V. Lorenzelli, J. Phys. Chem., 1990, 94, 8939–8945 CrossRef.
- M. L. Hair, Infrared Spectroscopy in Surface Chemistry, Marcel Dekker, New York, 1967, pp. 205–208 Search PubMed.
- I. Maupin, L. Pinard, J. Mijoin and P. Magnoux, J. Catal., 2012, 291, 104–109 CrossRef CAS.
- H. Pines and W. O. Haag, J. Am. Chem. Soc., 1960, 82, 2471–2483 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra08855d |
| This journal is © The Royal Society of Chemistry 2016 |