Development of highly-sensitive hydrazine sensor based on facile CoS2–CNT nanocomposites
Abstract
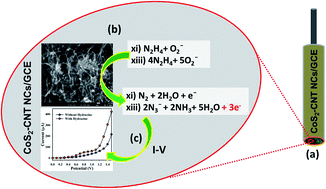
Cobalt pyrite-decorated carbon nanotube nanocomposites (CoS2–CNT NCs) were prepared by a simple wet-chemistry method in an alkaline medium. The characterization of the resulting CoS2–CNT NCs was performed in detail by field-emission scanning electron microscopy (FESEM), energy-dispersive spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), UV-vis spectroscopy, FT-IR spectroscopy, and X-ray diffraction patterns (XRD). A glassy carbon electrode (GCE) was fabricated from the CoS2–CNT NCs and developed into a chemical sensor for hydrazine (HZ) using a simple and reliable I–V method. The poisonous chemical HZ was selected as the target analyte in a selectivity study, which demonstrated the fast response of the GCE sensor fabricated from CoS2–CNT NCs using the I–V method. It also displayed an excellent sensitivity, a very low detection limit, long-term stability, and reproducibility. In a diagnostic study, a linear calibration plot (r2 = 0.9992) was obtained for a 0.1 nM to 1.0 mM aqueous solution of HZ, with a sensitivity of 4.430 μA nM−1 m−2 and low detection limit (LOD) of 0.1 nM. The potential of CoS2–CNT NCs in terms of chemical sensing is also discussed in this report. This approach is emerging as an effective technique for developing efficient chemical sensors for the detection of environmental pollutants in a large scale.

 Please wait while we load your content...
Please wait while we load your content...