Improved performance of a MnO2@PANI nanocomposite synthesized on 3D graphene as a binder free electrode for supercapacitors
Muhammad Asif*ab,
Yi Tana,
Lujun Pan*b,
Jiayan Lia,
Muhammad Rashadc,
Xin Fub,
Ruixue Cuib and
Muhammad Usmanb
aSchool of Materials Science and Engineering, Dalian University of Technology, Dalian 116024, PR China. E-mail: asifnust86@gmail.com
bSchool of Physics and Optoelectronic Technology, Dalian University of Technology, Dalian 116024, PR China. E-mail: lpan@dlut.edu.cn; Fax: +86 411 84709304
cCollege of Materials Science and Engineering, Chongqing University, Chongqing, 400044, PR China
First published on 4th May 2016
Abstract
In the current study, we have synthesized a binder free nickel foam graphene MnO2@PANI (MnO2@PANI/graphene) nanocomposite by simple hydrothermal method followed by in situ polymerization. The graphene grown on nickel foam is used as a 3D scaffold conducting substrate for binder free nanocomposite electrode synthesis. Morphological characterization of the nanocomposite reveals growth of the MnO2 intercalated PANI nanorods-like network. The electrochemical study demonstrates very interesting electrode activation phenomenon, i.e. increase in specific capacitance upto 1500 charge–discharge cycles. The activated MnO2@PANI/graphene nanocomposite exhibits a maximum specific capacitance of 1369 F g−1 at 3 A g−1 current density and excellent rate capability of more than 70% by varying current density from 3 A g−1 to 15 A g−1. The activated electrode displays good cyclic stability by retaining SC over 83% after 5000 cycles.
Introduction
The global energy crisis, threatening environmental concerns, and rapid development in portable electronics have raised increasing demands for highly efficient and renewable energy conversion/storage devices. The lithium ion batteries with high energy density and electrochemical capacitors (also called supercapacitors) with excellent power densities are the finest choices for energy storage applications.1 Among supercapacitors, electric double layer capacitors (EDLCs) which store charge through electrostatic interactions of ions at the electrolyte/electrode interface, suffer with low specific capacitance (SC), but exhibit high stability and long life cycle. The pseudocapacitors store charge based on faradaic reversible redox reactions at the active materials surface, and show high SC but suffer with low stability and short cyclic life.2The manganese dioxide is an outstanding pseudocapacitor material with outstanding electrochemical performance, excellent cycle life, environmental friendliness, and is cost effective. However, poor internal conductivity and lower cyclic stability make its experimental SC value much lower than that of theoretical value. Furthermore, agglomeration of MnO2 nanostructures results in lower surface area, consequently affecting electrochemical performance.2–4 The internal conductivity and agglomeration problems can be resolved by synthesizing nanocomposite with conducing polymers e.g., polyaniline (PANI), which itself is excellent pseudocapacitor material.5,6 Moreover, addition of binder in the nanocomposite material while preparing electrode for electrochemical characterization, significantly increases internal resistance of the composite electrode material, consequently losing electrochemical performance.7 Furthermore, direct growth of MnO2@PANI nanostructures on 3D conducting substrates (such as 3D graphene) can serve as binder free electrodes with considerably improve the SC and cyclic stability.
Graphene, a single layer of sp2 carbon atoms, has attracted fabulous research interest due to its unique optical, thermal, mechanical, electronic, and electrochemical properties, and thus wide range applications.8–12 The carbon nanostructures especially graphene has been considered as promising EDLCs electrode material, due to their versatile dimensionality, high aspect ratio, excellent electrical conductivity, and durable mechanical integrity.13–15 Unfortunately, EDLCs materials offer low specific capacitance. However, graphene can serve as 3D scaffold for pseudocapacitor nanostructures (e.g. MnO2 and polyaniline), thus can provide additional functions, e.g., excellent electrical conductivity, good cyclic stability, and enormous mass transport. Graphene/graphene oxide (GO), and CNTs have been used for the synthesis of binary/ternary nanocomposites with MnO2, PANI and other pseudocapacitor materials, using binder for electrode fabrication. However, there are very few reports on binder free growth of graphene based nanocomposites for supercapacitors.2,4,16–19
Recently, several attempts have been carried for synthesis of graphene–MnO2–PANI ternary nanocomposites using different methods, to enhance the SC of the electrode material. The high SC, long cycle life, and excellent rate capability of supercapacitors etc. are mainly determined by properties of active electrode materials, which in turn are greatly influenced by synthesized composite material's morphology, structure, and chemical composition.20,21 There are fewer reports on G/MnO2/PANI ternary nanocomposites and their electrochemical performances are not sufficiently good enough and require further improvement. Recently Han G. et al. reported 70% MnO2 nanorods coated with PANI ternary nanocomposite with GO (GO/MnO2/PANI) which exhibited highest SC of 512 F g−1, with 97% capacitance retention over 5000 cycles.22 In another work, Yu L. et al. reported synthesis of graphene/MnO2/PANI nanorods arrays ternary nanocomposite,23 which exhibited as high SC as 755 F g−1 at current density of 0.5 A g−1 and capacitance retention of 87% after 1000 cycles. Li K. et al. synthesized graphene/PANI/MnO2 ternary nanocomposite via oil–water interfacial polymerization approach.24 The synthesized nanocomposite exhibited max SC of 800 F g−1 at 0.4 A g−1 current density, with relatively low cyclic stability of 71% after 800 cycles, and good rate capability by retaining 505 F g−1 SC at 10 A g−1 current density. In another study, max SC for G/MnO2/PANI ternary nanocomposite is reported to be 875 F g−1 at 0.2 A g−1 in 1 M Na2SO4 electrolyte solution. The composite electrode retain 695 F g−1 SC when current density changes from 0.2 to 4 A g−1, showing good stability while retaining 93% of original SC after 1000 charge discharge cycles at 4 A g−1 current density.25
In current work we have used very simple ultrasonication assisted hydrothermal technique followed by in situ polymerization, to decorate MnO2@PANI nanostructures on 3D graphene which served as 3D conducting scaffold for binder free electrode. The synthesized nanocomposite revealed growth of MnO2 intercalated PANI nanorod like network morphology, thus limiting their agglomeration. Electrochemical characterization showed extraordinary electrochemical performance with significantly high SC value, good rate capability, and improved cyclic stability. Electrochemical activation of the composite electrode material was observed during SC measurements.
Experimental methods
Synthesis of MnO2@PANI/graphene nanocomposite
Chemical vapor deposition (CVD) was used to synthesize graphene on nickel foam (NFG) at high temperature 1010 °C. Nickel foam (420 g−2 area density and 1.6 mm thick) was rinsed via ultrasonication for 15 min in acetone, and was dried in N2 gas. The nickel foam template was heated to 1035 °C, and was annealed for 30 min under Ar/H2 (900/100 sccm) protection environment.For graphene growth, quartz tube was cooled down to 1010 °C, and Ar/H2/CH4 (1000/10/10 sccm) gaseous mixture was introduced for 15 min. After CVD reaction, the sample was allowed to cool down to room temperature in the Ar/H2 gaseous protection. Graphene mass on NF was estimated by the difference of mass of NF measured before and after the growth experiment.
The MnO2@PANI/graphene nanocomposite was synthesized by a simple green chemistry hydrothermal approach using ultrasonication for growth of MnO2 nanostructures, followed by in situ polymerization of polyaniline. The 0.02 M KMnO4 and 2.45 mM aniline solutions were prepared in DI water and were further subjected under ultrasonication for 10 min. For MnO2@PANI/graphene nanocomposite synthesis, 10 ml solution of each KMnO4 and aniline was mixed in a beaker and 2 × 2 cm2 NFG sample was dropped in the mixture solution. The solution mixture containing NFG template was further subjected to ultrasonication for 40 to 50 min, as a result the mixture's temperature was raised to 60–70 °C, subsequently leading to growth of MnO2 nanostructures. For in situ polymerization of aniline to polyaniline, 10 ml (1 mM) ammonium persulfate (APS) solution was poured slowly into the mixture solution, and then beaker was kept inside ice bath overnight. After in situ polymerization, the NFG sample coated with MnO2@PANI nanostructures was rinsed with DI water twice, and dried for 24 h at 60 °C. For electrochemical characterizations, electrode was prepared by sandwiching MnO2@PANI/graphene nanocomposite between two pieces of nickel foam and pressed under high pressure.
Characterizations
The morphological characterizations were performed by Field Emission Scanning Electron Microscopy (FE-SEM, NOVA NanoSEM450), and Transmission Electron Microscopy (TEM; FEI, Tecnai G2 F30S-Twin). Raman spectroscopy analysis was carried out using Renishaw in Via plus, operated at 632.8 nm, He–Ne laser, 4.0 mW laser power, and 50× objective lens. Crystallographic behavior was analyzed by X-Ray Diffraction (XRD, Cu Kα radiation, PANalytical B.V. Empyrean) with 2θ ranging from 10–90°. Elemental composition characterization was performed by X-ray Photoelectron Spectroscopy (XPS) VG ESCALAB 250 with an Al-Kα X-ray source operating at 150 W (15 kV). The calibration for binding energies was performed using C 1s peak at 284.6 eV, while the curve fitting was performed by XPS PEAK 4.1 software. Electrochemical characterizations were performed using conventional 3-electrode electrochemical cell (Modulab CHI660E) in 6 M KOH aqueous electrolyte solution, with platinum plate (20 × 20 mm2) as counter electrode and Ag/AgCl as reference electrode.Result and discussions
The graphene grown on nickel foam can serve as conducting 3D scaffold for growth of binder free MnO2@PANI nanocomposite electrode material. Fig. 1(a) depicts FESEM micrograph of the as grown NFG, which contain some wrinkles on its surface appeared during cooling process due to difference in coefficient of thermal expansion of graphene and nickel foam. The graphene grew uniformly on entire surface of nickel form, as illustrated in the low magnification FESEM image for NFG in the inset of Fig. 1(a). Fig. 1(b and c) illustrates low and high magnification micrographs for MnO2@PANI/graphene nanocomposite, respectively. The inset Fig. 1(b) illustrates the low magnification SEM image for MnO2@PANI/graphene nanocomposite, which clearly indicate that MnO2@PANI nanostructures were uniformly deposited on top and deeper part of NFG. It is obvious from the SEM micrographs that three different type of nanostructures exist in the nanocomposite, i.e. PANI nanosheets, agglomerated MnO2 nanostructures, and MnO2 intercalated PANI nanorods network. Previous studies have revealed that PANI nanorods morphologies show greater electrochemical performance. The PANI nanorods are either spread as branched network on the surface of NFG or in the form of small granular network (Fig. 1(c)). The intercalation of MnO2 in PANI nanorods was further confirmed by TEM image, as shown in Fig. 1(d) and SAED pattern in the inset. Second inset in (d) shows low magnification TEM image for branched like nanorods network. The detailed elemental analysis was performed by EDS color mapping from selected area of the sample, as shown in Fig. 2.The Fig. 2(a) illustrate the SEM image of the selected area for the EDS and mapping. The intercalation of MnO2 in the PANI nanorods is confirmed by EDS spectra, as depicted in Fig. 2(b). The high atomic percentage of carbon is due to PANI polymer and significant percentage of Mn is also observed, confirming intercalation of MnO2 in the PANI nanorods network. The EDS mapping for the area in Fig. 2(a) is illustrated in Fig. 2(c–e). The distribution of carbon element is almost uniform but is slightly higher at MnO2@PANI hybrid structures due to polymer carbon, as shown in Fig. 2(c). Similarly, the oxygen element distribution is higher on hybrid nanostructures area, which could be due to intercalation of MnO2 on PANI nanostructures and agglomeration of MnO2 nanostructures as well, as illustrated in Fig. 2(d). The Mn element is uniformly distributed over PANI nanosheets, nanorods network, and MnO2 agglomeration areas, which confirm intercalation/coating of MnO2 inside or over PANI nanostructures, as shown in Fig. 2(e).
 | ||
| Fig. 2 Elemental analysis for MnO2@PANI/graphene nanocomposite: (a) SEM image, (b) EDS spectra, and (c–e) EDS mapping taken from (a) for carbon, oxygen, and Mn elements, respectively. | ||
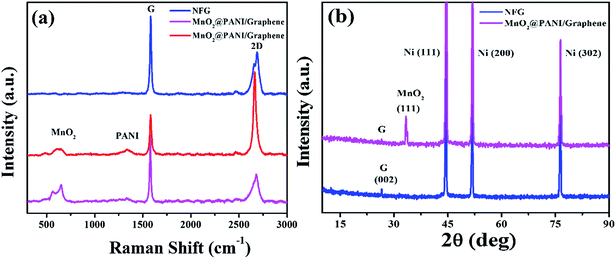
The Raman spectroscopy and X-ray diffraction (XRD) measurements were carried out for NFG and MnO2@PANI/graphene nanocomposite electrode material. Fig. 3(a) depicts Raman spectroscopic spectra for NFG and MnO2@PANI/graphene nanocomposite. The Raman spectra for pristine graphene grown on Ni foam, comprises of two strong peaks for G and 2D bands at 1582 cm−1 and 2686 cm−1, respectively. The defect related D band is missing from the spectra confirming growth of defect free and high quality graphene on the Ni foam. The 2D to G bands intensity ratio for Raman spectra taken at different positions of the NFG revealed growth of multilayer graphene. However, few places we also get spectra for mono or bilayer graphene. Raman spectra for MnO2@PANI/graphene nanocomposite contains three additional peaks at 490, 585, and 660 cm−1 along with G and 2D bands, which confirm the growth of MnO2 nanostructures. The intensity of MnO2 peaks was stronger at densely covered MnO2@PANI nanostructures due to which G and 2D bands disappear in those spectra (not shown over here). However, peaks for PANI nanostructures are of low intensity and are further suppressed due to relatively high intensity of G and 2D bands for graphene.
To support Raman spectroscopic results, XRD study is performed. The crystalline phase purity and composition of NFG and MnO2@PANI/graphene nanocomposite are illustrated in Fig. 3(b). The XRD spectrum for the pristine graphene grown on nickel foam illustrates single diffraction peak originating at angle 2θ equal to 26.5° corresponding to (002) reflection of graphene, in addition to the three peaks originated from Ni at 44.5°, 51.8°, & 76.4°. The XRD spectra for MnO2@PANI/graphene nanocomposite depicts additional peak at angle 2θ equal to 33.3° confirming growth of the MnO2 nanostructures, along with strong peaks for Ni and weak peak for graphene. The diffraction peak for graphene is not obvious due to low quantity in form of few layer graphene thin film.
For elemental characterizations of the synthesized composite were further confirmed by X-ray photoelectron spectroscopy (XPS). The wide scan survey spectra shows Mn 2p, O 1s, N 1s, and C 1s peaks, which confirm the growth of MnO2@PANI nanostructures, as illustrated in Fig. 4(a). The spectra for Mn 2p have two peaks corresponding to Mn 2p1/2 and Mn 2p3/2 at binding energy 642.85 eV and 654.5 eV, respectively (Fig. 4(b)).
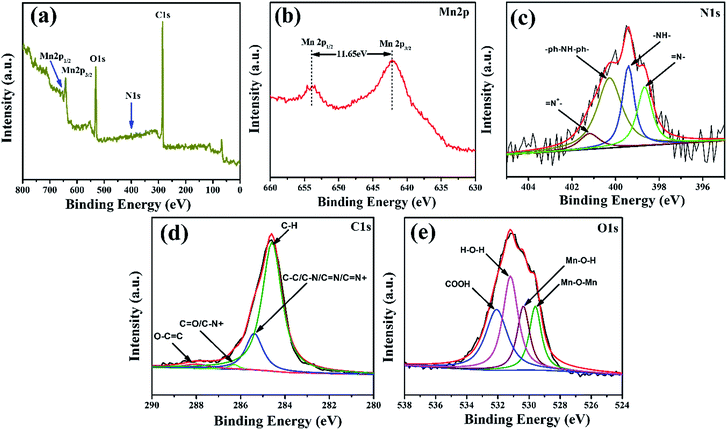 | ||
| Fig. 4 X-ray photoelectron spectroscopy (XPS) analysis of the composite: (a) survey spectra, (b) Mn 2p spectra, (c) N 1s spectra, (d) C 1s spectra, and (e) O 1s spectra. | ||
The BE difference between Mn 2p1/2 and Mn 2p3/2 peaks is 11.65 eV, (almost equal to 11.7 eV) which is characteristics for MnO2 nanostructures.26 Fig. 4(c) depicts N 1s XPS spectra, which is further deconvoluted into four sub peaks at binding energies 398.67, 399.43, 400.27, and 401.23 eV which correspond to imine like [![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–], amine [–NH–], [–ph–NH–ph–], and protonated imine [
N–], amine [–NH–], [–ph–NH–ph–], and protonated imine [![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+–], respectively.27,28 The intrinsic oxidation state for PANI may change from fully oxidized pernigraniline state, through half oxidized emeraldine state, to fully reduced leucoemeraldine state. The ultimate conductive state for PANI nanostructures can be realized either by protonation of imine nitrogen (–N–) in its emeraldine state or through oxidation of the amine nitrogen (–NH–) in completely reduced leucoemeraldine state.29
N+–], respectively.27,28 The intrinsic oxidation state for PANI may change from fully oxidized pernigraniline state, through half oxidized emeraldine state, to fully reduced leucoemeraldine state. The ultimate conductive state for PANI nanostructures can be realized either by protonation of imine nitrogen (–N–) in its emeraldine state or through oxidation of the amine nitrogen (–NH–) in completely reduced leucoemeraldine state.29
PANI nanostructures in the composite are in half oxidation and half reduction state, which are further confirmed by electrochemical characterization. The C 1s peak for the synthesized composite can be decomposed into four component peaks, at binding energy 284.51 eV, 285.08 eV, 286.36 eV, and 287.92 eV, which represent aromatic C–H, carbon atom bonded to carbon or nitrogen atoms C–C/C–N/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+, carbonyl C
N+, carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O surface functional group, and the carboxyl O–C
O surface functional group, and the carboxyl O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O surface functional group of graphene electrode, respectively.28,30 Moreover, the peak at 284.51 eV is due to the sp2 carbon atoms in graphene, and C–C bonds vibrations in the benzenoid rings, which is back bone of polymer. The peak at 285.08 eV might be appeared due to bonding of carbon to carbon neutral atoms, or nitrogen atoms, which also confirm the existence of PANI. The existence of MnO2 can be further varied by O 1s peaks, which is further deconvoluted into four component peaks. The peaks centered at BE 529.64 eV, 530.49 eV, 531.28 eV, and 532.30 eV are corresponding to Mn–O–Mn, Mn–O–H, H–O–H, and COOH bonds vibrations, respectively.27,31,32 The peak at 532.30 eV is due to the COOH functional group attached to the basal graphene.
O surface functional group of graphene electrode, respectively.28,30 Moreover, the peak at 284.51 eV is due to the sp2 carbon atoms in graphene, and C–C bonds vibrations in the benzenoid rings, which is back bone of polymer. The peak at 285.08 eV might be appeared due to bonding of carbon to carbon neutral atoms, or nitrogen atoms, which also confirm the existence of PANI. The existence of MnO2 can be further varied by O 1s peaks, which is further deconvoluted into four component peaks. The peaks centered at BE 529.64 eV, 530.49 eV, 531.28 eV, and 532.30 eV are corresponding to Mn–O–Mn, Mn–O–H, H–O–H, and COOH bonds vibrations, respectively.27,31,32 The peak at 532.30 eV is due to the COOH functional group attached to the basal graphene.
Electrochemical performance of MnO2@PANI/graphene nanocomposite electrode material
The MnO2@PANI/graphene nanocomposite synthesized by ultrasonication assisted hydrothermal in situ polymerization provide a practical pathway for highly efficient electrode material for energy storage devices. The structural characterizations of the nanocomposite revealed the growth of MnO2 intercalated PANI nanorods like branched network. This may shorten electrolyte ion diffusion path and may result in deep redox reaction, subsequently boosting electrochemical performance of the electrode. The cyclic voltammetry (CV), galvanostatic charge–discharge (CD), life cycle, and electrochemical impedance spectroscopy (EIS) tests were executed in conventional three-electrode cell, to estimate electrochemical performance of the nanocomposite as binder free electrode for supercapacitors. The CV tests were performed in a potential window of 0.05 to 0.35 V vs. current, at varying scan rate from 1 to 100 mV s−1 in 6 M KOH electrolyte solution (Fig. 5 & 6). | ||
| Fig. 6 The cyclic stability of the MnO2@PANI/graphene nanocomposite (a), and Nyquist plot for activated nanocomposite electrode (b). | ||
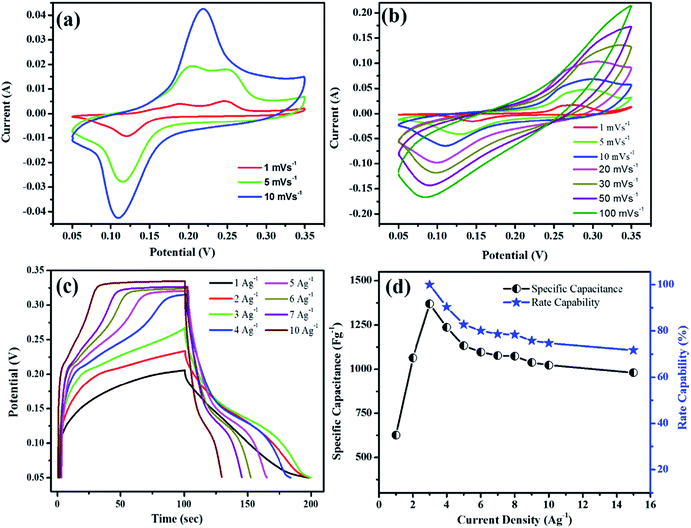
The synthesized MnO2@PANI/graphene nanocomposite exhibited exceptional electrochemical performance. The cyclic voltammetry SC measurements for the nanocomposite electrode material were performed at different scan rates, as illustrated in Fig. 5(a and b). The SC from CV curves can be calculated using the following relation.
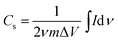 | (1) |
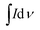 is the integrated area of the CV curve, m is the active mass of electrode material, ΔV is the potential range, and ν is the scan rate. It is quite interesting to note that CV curves measured after first cycle show two distinct redox peaks, as illustrated in Fig. 5(a). However, CV curves measured after 1500 galvanostatic charge discharge cycles show relatively symmetric curves with single redox peak at low scan rate which became broad at higher sane rates Fig. 5(b).
is the integrated area of the CV curve, m is the active mass of electrode material, ΔV is the potential range, and ν is the scan rate. It is quite interesting to note that CV curves measured after first cycle show two distinct redox peaks, as illustrated in Fig. 5(a). However, CV curves measured after 1500 galvanostatic charge discharge cycles show relatively symmetric curves with single redox peak at low scan rate which became broad at higher sane rates Fig. 5(b).
Moreover, the redox peaks show slight shift with the increase in scan rate which might be attributed to the increase in internal resistance of the electrolyte ions, therefore resulting in decline of electrochemical performance. This might be attributed to the transition of PANI from one state to the other state. It is obvious from the CV curves that nanostructures can conduct electron and ions at higher scan rates, which can be verified by significant increment in current at higher scan rates.33,34 Thus the nanocomposite material at first cycle is termed as non-activated electrode while after 1500 CD cycles it is referred as activated electrode. The activated nanocomposite electrode showed high cyclic voltammetry SC values, i.e. 2731, 1712, 1305, 850, 715, 594, and 423 F g−1 at 1, 5, 10, 20, 30, 50, and 100 mV s−1 scan rates, respectively. The galvanostatic charge–discharge (CD) measurements for SC at different current densities for the activated MnO2@PANI/graphene nanocomposite electrode are illustrated in the Fig. 5(c and d). The SC from galvanostatic CD curves can be measured using the following relation.
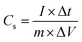 | (2) |
The activation process and cyclic stability of the synthesized nanocomposite are further analyzed, as shown in the Fig. 6(a). The non-activated nanocomposite electrode (at first cycle) exhibited maximum galvanostatic SC of 809 F g−1 at 2.5 A g−1 current density. Interestingly, the SC of the electrode increases significantly with the increase in CD cycles. The maximum SC 1369 F g−1 was achieved at 3.0 A g−1 current density after 1500 cycles, and this process is referred as activation of the nanocomposite electrode material. This increase in SC value is much more significant compared to reported work in the literature. There might be several possible reasons for this phenomenon. One of the possible reasons is transition of PANI from incomplete oxidation state to its most stable state, which is verified by alteration of double redox peaks CV curves for non-activated electrode to the symmetric CV curves for activated electrode (Fig. 5(a and b)). Moreover, electrolyte might have taken some time to penetrate deep into the micro/mesopores of composite electrode nanostructures, widening or creating additional micro/mesopores.38 This could have caused deep faradaic redox reactions resulting in high electrochemical performance. To completely understand this phenomenon, additional in-depth research is required, which we will carry out in future. The SC of the activated electrode decreases to 1136 F g−1 at 3.0 A g−1 after 5000 cycles, alternatively the activated electrode retained about 83% of SC after 5000 CD cycles.
The electrochemical impedance spectroscopy (EIS) analysis reveals low internal resistance of the activated electrode, as depicted in Fig. 6(b). The plot shows very small diameter semicircle at high frequency with straight vertical inclined, demonstrating the low internal resistance and excellent SC of the synthesized electrode material. The inset is wide frequency range Nyquist plot for the MnO2@PANI/graphene nanocomposite electrode material. Significantly low internal or equivalent series resistance of the activated electrode might be attributed to the highly conducting substrate i.e., 3D graphene. The MnO2@PANI nanostructures grew on 3D graphene scaffold which reduce ion diffusion path during electrochemical measurements. Furthermore, electric charge can easily transmit from the MnO2@PANI nanostructures to the graphene substrate, and thus cause very low equivalent series resistance of the electrode material.
Conclusions
The MnO2@PANI/graphene nanocomposite was synthesized using simple ultrasonication assisted hydrothermal growth of MnO2 nanostructures, followed by in situ polymerization of PANI. The morphological study revealed growth of MnO2 intercalated PANI nanorod like network structures which boosted electrochemical performance of the electrode. The electrochemical characterization revealed the increase in specific capacitance of the MnO2@PANI/graphene nanocomposite electrode from 809 F g−1 to 1369 F g−1 with increasing charge–discharge cycles up to 1500. This could be attributed to the penetration of electrolyte deep into the inherent pores of the active electrode material and may widen or create new micro/mesopores, resulting in deep faradaic redox reactions.Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 11274055 and 61137005), the Program for Liaoning Excellent Talents in University, and Chinese Scholarship Council (CSC).Notes and references
- Y. Tian, S. Cong, W. Su, H. Chen, Q. Li, F. Geng and Z. Zhao, Nano Lett., 2014, 14, 2150–2156 CrossRef CAS PubMed.
- J.-G. Wang, F. Kang and B. Wei, Prog. Mater. Sci., 2015, 74, 51–124 CrossRef CAS.
- J. Mei and L. Zhang, Electrochim. Acta, 2015, 173, 338–344 CrossRef CAS.
- M. Huang, R. Mi, H. Liu, F. Li, X. L. Zhao, W. Zhang, S. X. He and Y. X. Zhang, J. Power Sources, 2014, 269, 760–767 CrossRef CAS.
- A. Sumboja, C. Y. Foo, J. Yan, C. Yan, R. K. Gupta and P. S. Lee, J. Mater. Chem., 2012, 22, 23921–23928 RSC.
- L. Ren, G. Zhang, J. Wang, L. Kang, Z. Lei, Z. Liu, Z. Liu, Z. Hao and Z. H. Liu, Electrochim. Acta, 2014, 145, 99–108 CrossRef CAS.
- H. P. Cong, X. C. Ren, P. Wang and S. H. Yu, Energy Environ. Sci., 2013, 6, 1185–1191 CAS.
- M. Asif, Y. Tan, L. Pan, J. Li, M. Rashad and M. Usman, J. Phys. Chem. C, 2015, 119, 3079–3089 CAS.
- L. Liao, Y.-C. Lin, M. Bao, R. Cheng, J. Bai, Y. Liu, Y. Qu, K. L. Wang, Y. Huang and X. Duan, Nature, 2010, 467, 305–308 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- M. Rashad, F. Pan, A. Tang, M. Asif, S. Hussain, J. Gou and J. Mao, J. Ind. Eng. Chem., 2015, 23, 243–250 CrossRef CAS.
- M. Rashad, F. Pan, A. Tang and M. Asif, Progress in Natural Science: Materials International, 2014, 24, 101–108 CrossRef.
- Q. Li, N. Mahmood, J. Zhu, Y. Hou and S. Sun, Nano Today, 2014, 9, 668–683 CrossRef CAS.
- C. Z. Yuan, B. Gao, L. F. Shen, S. D. Yang, L. Hao, X. J. Lu, F. Zhang, L. J. Zhang and X. G. Zhang, Nanoscale, 2011, 3, 529–545 RSC.
- X.-F. Jiang, X.-B. Wang, P. Dai, X. Li, Q. Weng, X. Wang, D.-M. Tang, J. Tang, Y. Bando and D. Golberg, Nano Energy, 2015, 16, 81–90 CrossRef CAS.
- F. Li, X. Jiang, J. Zhao and S. Zhang, Nano Energy, 2015, 16, 488–515 CrossRef CAS.
- H. Mi, J. Zhou, Q. Cui, Z. Zhao, C. Yu, X. Wang and J. Qiu, Carbon, 2014, 80, 799–807 CrossRef CAS.
- C. Gao and G. Chen, Compos. Sci. Technol., 2016, 124, 52–70 CrossRef CAS.
- M. Khalid, M. A. Tumelero and A. A. Pasa, RSC Adv., 2015, 5, 62033–62039 RSC.
- M. Vangari, T. Pryor and L. Jiang, J. Energy Eng., 2013, 139, 72–79 CrossRef.
- E. Hosono, S. Fujihara, I. Honma, M. Ichihara and H. Zhou, J. Power Sources, 2006, 158, 779–783 CrossRef CAS.
- G. Han, Y. Liu, L. Zhang, E. Kan, S. Zhang, J. Tang and W. Tang, Sci. Rep., 2014, 4, 4824 Search PubMed.
- L. Yu, M. Gan, L. Ma, H. Huang, H. Hu, Y. Li, Y. Tu, C. Ge, F. Yang and J. Yan, Synth. Met., 2014, 198, 167–174 CrossRef CAS.
- K. Li, D. Guo, J. Chen, Y. Kong and H. Xue, Synth. Met., 2015, 209, 555–560 CrossRef CAS.
- C. Pan, H. Gu and L. Dong, J. Power Sources, 2016, 303, 175–181 CrossRef CAS.
- C. Zhu, S. Guo, Y. Fang, L. Han, E. Wang and S. Dong, Nano Res., 2011, 4, 648–657 CrossRef CAS.
- L.-J. Sun, X.-X. Liu, K. K.-T. Lau, L. Chen and W.-M. Gu, Electrochim. Acta, 2008, 53, 3036–3042 CrossRef CAS.
- M. Zhong, Y. Song, Y. Li, C. Ma, X. Zhai, J. Shi, Q. Guo and L. Liu, J. Power Sources, 2012, 217, 6–12 CrossRef CAS.
- P. C. Rodrigues, M. Muraro, C. M. Garcia, G. P. Souza, M. Abbate, W. H. Schreiner and M. A. B. Gomes, Eur. Polym. J., 2001, 37, 2217–2223 CrossRef CAS.
- S. W. Lee, B.-S. Kim, S. Chen, Y. Shao-Horn and P. T. Hammond, J. Am. Chem. Soc., 2009, 131, 671–679 CrossRef CAS PubMed.
- R. K. Sharma, A. C. Rastogi and S. B. Desu, Electrochim. Acta, 2008, 53, 7690–7695 CrossRef CAS.
- S. C. Ray, A. Saha, S. K. Basiruddin, S. S. Roy and N. R. Jana, Diamond Relat. Mater., 2011, 20, 449–453 CrossRef CAS.
- L. Huang, D. Chen, Y. Ding, S. Feng, Z. L. Wang and M. Liu, Nano Lett., 2013, 13, 3135–3139 CrossRef CAS PubMed.
- X. Yu, B. Lu and Z. Xu, Adv. Mater., 2014, 26, 1044–1051 CrossRef CAS PubMed.
- Q. Lu, J. G. Chen and J. Q. Xiao, Angew. Chem., Int. Ed., 2013, 52, 1882–1889 CrossRef CAS PubMed.
- J. Chmiola, C. Largeot, P.-L. Taberna, P. Simon and Y. Gogotsi, Angew. Chem., Int. Ed., 2008, 47, 3392–3395 CrossRef CAS PubMed.
- J. Ji, L. L. Zhang, H. Ji, Y. Li, X. Zhao, X. Bai, X. Fan, F. Zhang and R. S. Ruoff, ACS Nano, 2013, 7, 6237–6243 CrossRef CAS PubMed.
- X. Li, L. Yang, Y. Lei, L. Gu and D. Xiao, ACS Appl. Mater. Interfaces, 2014, 6, 19978–19989 CAS.
| This journal is © The Royal Society of Chemistry 2016 |