Synthesis and characterization of benzilic alcohol metalloporphyrin and its nanocomposite with graphene oxide (GO–CoTHMP) and investigation of their efficiency in the removal of environmental pollutants
Abstract
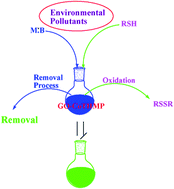
5,10,15,20-Tetrakis-(4-hydroxymethylphenyl)porphyrin (THMP), metalloporphyrin [(Co(III)THMP)] and its nanocomposites with graphene oxide (GO–CoTHMP) were synthesized for the first time. The structure of synthesized GO–CoTHMP was characterized by FT-IR, UV-Vis, X-ray powder diffraction (XRD), and scanning electron microscopy (SEM) and AFM was used to assess the distances between layers in the nanocomposite. The efficiency of GO–CoTHMP in the removal of environmental contaminants was tested by degradation of methylene blue and oxidation of thiophenol, two important pollutants. The synthesized nanocomposite shows excellent selectivity in the oxidation of thiophenol and high adsorption capacity for MB and may be an effective catalyst for the removal of these environmental pollutants.

 Please wait while we load your content...
Please wait while we load your content...