Development of an integrated system for the treatment of rural domestic wastewater: emphasis on nutrient removal
Prangya Ranjan Rout,
Rajesh Roshan Dash and
Puspendu Bhunia*
School of Infrastructure, Indian Institute of Technology Bhubaneswar, Odisha, India 751 013. E-mail: pbhunia@iitbbs.ac.in; Fax: +91-674-2301983; Tel: +91-674-2306355
First published on 13th May 2016
Abstract
With the aim of enhancing the nutrient removal from rural domestic wastewater while reducing the cost of the treatment process, a novel, integrated treatment system consisting of a multi-stage bio-filter with drop aeration and a post positioned attached growth carbonaceous denitrifying bio-reactor was designed and developed in this study. The bio-filter was packed with ‘dolochar’, a sponge iron industry waste, as an adsorbent mainly for phosphate removal through a physicochemical approach. The denitrifying bio-reactor was packed with many waste organic solid substances (WOSS) as carbon sources and substrates for biomass attachment, mainly to remove nitrate in the biological denitrification process. The performance of the modular system, treating real domestic wastewater was monitored for a period of about 60 days and the average removal efficiencies during the period were as follows: phosphate, 99.48%; nitrate, 92.44%, ammonia, 96.64%, with mean final effluent concentration of 0.153, 5.5, and 1.06 mg L−1, respectively. This treatment system would allow multipurpose reuse of the final effluent. Moreover, the saturated dolochar can be used as a nutrient supply in agricultural practices and the partially degraded carbonaceous substances can also be used as an organic fertilizer after composting. Thus, the system displays immense potential for treating domestic wastewater significantly by decreasing the concentrations of nutrient and most importantly, facilitating the conversion of the waste materials into usable ones.
1. Introduction
Over the past few decades, many government policies of developing nations like India are putting emphasis on the rapid advancement of rural and peri-urban areas. With the aim of upgrading the living standards of the mentioned areas, many developmental activities are being undertaken in an accelerated manner. As a result, excess generation of wastewater is taking place through various anthropogenic activities and subsequently discharged to the environment due to a lack of proper management of sewage in those areas. Moreover in rural areas, it is a common practice to discharge the domestic wastewater into surface water bodies without efficient and proper treatment processes.1,2 These are the two major causes of the deterioration of water quality in rural areas, and the domestic wastewater is the dominant cause and the main contributor of inorganic nutrient (ammoniacal nitrogen, nitrate nitrogen, and orthophosphate) to the aquatic environment.3 So the increased incidence of eutrophication which is a global widespread problem in recent times, is primarily due to the input of nutrient from rural domestic wastewater.4 Moreover, the detrimental impacts of nutrient overloading apart from eutrophication are becoming gradually more noticeable in the form of production of algal toxins, impairment of human health, reduced biodiversity of aquatic species, impacts on global nutrient salvaging, and increased treatment costs etc.5–7 Likewise, the presence of nutrient in wastewater speed up the production of microcystin, a toxin that can cause hepatocellular carcinoma in humans and the formation of carcinogenic compounds like nitrosamines and nitrosamides.8,9 Therefore, nutrient inputs must be reduced and measures for effective reduction of nutrient concentrations in domestic wastewater must be developed to protect the aquatic ecosystems from deterioration. As a matter of fact, growing attention is being paid these days to the development of appropriate technologies for domestic wastewater treatment and subsequent improvement of effluent quality with respect to the nutrient in rural areas.Dispersed and horizontal distribution of the population in rural areas makes collection and treatment of rural domestic wastewater problematic.4 Conventional centralized treatment systems which are operated in large and small cities require considerable investments in their construction and operation. So in a developing country like India, lack of economic resources, lack of local expertise, prohibitive construction costs, and dispersed populations, make centralized wastewater treatment system impractical in rural areas. Additionally, due to the inherent variability of domestic wastewater characteristics like quality, quantity, composition etc. the centralized treatment systems are often not suitable for rural wastewater treatment.1,4 On the other hand, as stated in the previous paragraph, most rural areas are experiencing a major water contamination threat resulting from the untreated or improper treatment of domestic wastewater. There is a pressing need to develop dependable technologies that are of simple design, low cost, energy extensive, employ non-sophisticated equipment, support low maintenance and highly efficient for the treatment of widely distributed rural domestic wastewater in developing countries. The decentralized treatment systems, characterized in collecting, treating, disposing and reusing onsite, thereby lowering construction and operational costs by avoidance of extensive collecting network, appear to be a much better choice for rural areas.10 Therefore, it is indispensable to look for unconventional onsite or decentralized wastewater treatment systems for rural regions that can fulfill the criteria of affordability, sustainability, and environmental acceptability.
Some low-cost alternative onsite treatment solutions for decentralized treatment of rural domestic wastewater have been developed and operated are constructed soil filters (CSFs), intermittent sand filters (ISF), land treatment systems (LTS) soil infiltration trenches, vermin filters (VFs), high rate algal ponds, compound media filter bed combined systems, etc.4,11–16 However, many of them are focused on removal of organic pollutants and are not optimized for nutrient removal. In the due course of time, membrane bio-reactors, aerobic biological treatment systems, constructed wetlands (CWs), etc. have been promoted in rural wastewater treatment emphasizing on nutrient removal.17–19 Though aerobic biological treatment processes and membrane bio-reactors are competent enough in nutrient removal but their complex operation, fouling associated with the membrane, energy intensive aeration, cost incurred in membrane, etc., add to the non-affordability of the systems in rural regions.1 However, the use of CWs has turn out to be the most commonly practicing treatment technology worldwide for onsite treatment of domestic wastewater because of their low operating cost, good treatment efficiency, and easy maintenance. Vertical flow CWs nitrify but they do not denitrify properly, while horizontal flow CWs denitrify but they do not nitrify properly.20,21 Overall, the removal of nutrient in CWs is generally inadequate possibly due to unavailability of carbon source or because of the lack of necessary oxygen to oxidize ammonium or due to absence of proper anoxic condition or sometimes due to the low sorption capacity of ordinary substrates utilized for phosphate withholding.21 Though hybrid CWs with an appropriate substrate for phosphate retention can solve these problems but those are not been used too often for decentralized treatment of rural domestic wastewater.22 From previous studies, it can be inferred that a single treatment system may not be able to remove nutrient completely from rural domestic wastewater. If some systems are efficient in phosphate removal they may not exhibit equal efficiency in case of nitrate or ammonium nitrogen removal and vice versa. Therefore, the research focus has been shifted to development of hybrid or combined systems, where the advantages of individual systems can be realized to complement each other in order to get nutrient free effluent.21
On-site systems using media filters have emerged as a promising solution for nutrient removal and are of particular interest for phosphate removal.23 The mechanism of phosphate removal in filter techniques is based on the fundamental of adsorption and precipitation within the formulated filter materials. Adsorption is one of the promising physicochemical approaches for the elimination of phosphate and could be easily applied to on-site treatment facilities without yielding any harmful by-products.24 Therefore, the interest is growing for the trickling bio-filters employing appropriate and efficient media materials to facilitate physicochemical phosphate removal. On the other hand, biological nitrification–denitrification is the principal process that has been demonstrated to be feasible, both economically and technically, for nitrogen removal. This conventional process includes a two-stage, sequential aerobic-anaerobic treatment system, wherein the first step ammonia gets converted to nitrate through nitrite in the presence of oxygen (autotrophic nitrification) and subsequently denitrified to gaseous nitrogen under anoxic conditions when nitrate is used as an electron acceptor for microbial respiration (heterotrophic denitrification).25 There are evidences of using tricking filters for removal of ammonia, whereas denitrifying bio-reactors, or bio-filters, are considered cost effective and emerging technologies for nitrate removal.26,27 The majority of previous studies are available on either utilization of physicochemical process for phosphorous removal or biological process for removal of nitrogen from wastewaters, but no comprehensive report is available on utilizing potentials of both the systems to develop an effective integrated system, comprising of biological nitrogen removal combined with physicochemical phosphorous removal for simultaneous nutrient removal from domestic wastewater of rural areas.28 By choosing a suitable packing material for trickling bio-filter that may facilitate nitrification and phosphate accumulation and subsequently integrating the same with a denitrifying bio-reactor, a simple, sustainable, decentralized wastewater treatment system for nutrient removal can be realized.
Providing simple, reliable and affordable nutrient removal facilities in rural areas is a challenge, particularly in developing countries. So in this work, an attempt has been made to design and develop a novel integrated treatment system to serve the purpose. The designed hybrid system consists of a multi-stage trickling bio-filter and a post positioned denitrifying bio-reactor. The system ideally removes nitrogen and phosphorous simultaneously at reduced treatment costs through the use of innovative techniques like unique combination of trickling bio-filter and denitrifying bio-reactor, a new packing material, a sponge iron industry waste dolochar, for phosphate retention, drop aeration for efficient nitrification, use of waste organic solid substances (WOSS) as carbon source as well as provide surface area for biomass attachment in the denitrifying bio-reactor. Dolochar packed multi-stage trickling bio-filter adsorbs phosphate and enables oxygenation by drop aeration techniques, thereby transforming ammonium to nitrate by nitrification. The complete nitrogen removal by denitrification takes palace in the denitrifying bio-reactor. The entire set-up works on the principle of attached growth process that offers several advantages over the contemporary technologies involving suspended biomass like stability towards variability in domestic wastewater composition and flow, reducing bio-reactor size, high hydraulic loading rate, etc., thus, can outperform other existing treatment technologies for nutrient removal. Other several uniqueness as offered by the system includes (i) use of waste materials (dolochar and WOSS) for the treatment of wastewater, so cost effective, (ii) drop aeration without involvement of mechanical device, so energy extensive, (iii) compact design, so reduction of system footprint, (iv) modular design, scaling-up is easy, (v) no harmful by products from the system, so environmental friendly, (vi) exhausted dolochar can be used in agricultural sector as a source of nutrient and degraded WOSS can be utilized as organic fertilizer after subjecting it to composting, so enabling conversion of waste into wealth. Thus to gain a more thorough understanding of the nutrient removal processes in the novel integrated system, the present study is conducted with the following objectives: (i) to evaluate the performance efficiency of the integrated system for nutrient removal by using real domestic wastewater, (ii) to evaluate the stability of the system performance under various experimental conditions, (iii) to assess the oxygen transfer capability of the drop aeration techniques, (iii) to study the effect of influent nutrient concentration on removal efficiency, (iv) to figure out efficacy of dolochar and WOSS and (v) to establish the applicability of the spent adsorbent as a nutrient supplier.
2. Materials and methods
2.1. Experimental set-up
Many laboratory scale integrated systems were fabricated in the laboratory using poly-acrylic sheets. Fig. 1a represents the schematic diagram of the designed experimental set-up with all the dimensional details whereas Fig. 1b illustrates the process flow of a single representative integrated system which consists of a multi-stage trickling bio-filter followed by a denitrifying bio-reactor. The trickling bio-filter consisted of three to five identical units each having a dimension of 25 cm × 12 cm × 5 cm (l × b × h). Each unit was partitioned into two segments. The first segment with a dimension of 2.5 cm × 12 cm × 3 cm was to receive the incoming wastewater and the second segment with a dimension of 22.5 cm × 12 cm × 5 cm was the filter bed packed with the industrial waste, dolochar as bed material. The water passed from the first segment to the second one by overflowing through the partition, thereby facilitating uniform distribution throughout the bed. The packing was done to a height of 2.5 cm, so making the total volume approximately 675 mL (working volume of 400 mL, considering 60% porosity of the bed) and a surface area of 270 cm2. The base of each unit was sloped 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 towards the end in an alternative fashion to direct the flow of water along the bed under the influence of gravity. A gap of 20 cm was maintained in between the filtration unit to realize oxygenation by drop aeration method. Similarly, the denitrifying bio-reactor was having the geometry of 30 cm × 30 cm × 4 cm (l × b × h). The bio-reactor was segmented into five compartments and each one having a width of 4.5 cm, length and breadth being the same as mentioned earlier formed a packed bed using WOSS as a carbon source and media to support bio-film formation. Each compartment was separated by baffles and there were gaps of 1.25 cm in between two baffles. The baffles inside the reactor were used to direct the flow of wastewater along the compartments in a zigzag manner. The overall volume of the bio-reactor was something like 2.5 L and considering bed porosity of around 80% after packed with WOSS, the working volume was reduced to roughly 2.0 L. The WOSS was packed up to a height of 3.5 cm and leaving 0.5 cm of height corresponding to a volume more or less 0.45 L for the accumulation of gas. The bio-reactor was made airtight to facilitate anoxic condition and there was a regulator on the top surface of the anoxic chamber for routine monitoring and periodic release of accumulated gases to the atmosphere.
25 towards the end in an alternative fashion to direct the flow of water along the bed under the influence of gravity. A gap of 20 cm was maintained in between the filtration unit to realize oxygenation by drop aeration method. Similarly, the denitrifying bio-reactor was having the geometry of 30 cm × 30 cm × 4 cm (l × b × h). The bio-reactor was segmented into five compartments and each one having a width of 4.5 cm, length and breadth being the same as mentioned earlier formed a packed bed using WOSS as a carbon source and media to support bio-film formation. Each compartment was separated by baffles and there were gaps of 1.25 cm in between two baffles. The baffles inside the reactor were used to direct the flow of wastewater along the compartments in a zigzag manner. The overall volume of the bio-reactor was something like 2.5 L and considering bed porosity of around 80% after packed with WOSS, the working volume was reduced to roughly 2.0 L. The WOSS was packed up to a height of 3.5 cm and leaving 0.5 cm of height corresponding to a volume more or less 0.45 L for the accumulation of gas. The bio-reactor was made airtight to facilitate anoxic condition and there was a regulator on the top surface of the anoxic chamber for routine monitoring and periodic release of accumulated gases to the atmosphere.
 | ||
| Fig. 1 (a) Schematic diagram of the integrated system, (b) process flow of a representative integrated system. | ||
2.2. Filter bed materials
A sponge iron industry solid waste, dolochar is used as the filter bed material in this study. Dolochar samples were collected from different sponge iron industries situated in Odisha, India. The collected samples were subjected to grinding, sieving, followed by washing several times with distilled water to remove surface adhered particles, soluble materials and then dried in a hot-air oven at 100 °C for overnight. Particle sizes ranging from 0.3 mm to 4.2 mm were used as the bed material in this study.2.3. Description of carbon source and bio-film carrier
The selection of the waste organic solid substances was done on the basis of their extensive availability and no or low cost. After preliminary experiments mixture of three waste organic solid substances named as sugarcane baggage (SB), groundnut shell (GS), coconut shell (CS) were selected as potential carbon sources and substrate for bio-film attachment to facilitate denitrification process. Since, mixtures of multiple organic sources may support a wider consortium of microbes and may, thus, show higher microbial activities than that of single sources.29 All the WOSS were collected from Bhubaneswar city of Odisha, India. The solid wastes are rough textured with irregularity in shape. The collected samples were washed with water several times to remove surface adhered particles, soluble materials and then dried under laboratory conditions at around 60 °C for overnight. Then the dried samples were shredded, sieved and the particle sizes between 0.6 to 4.75 mm were selected and stored at room temperature in a moisture-free container for further use.2.4. Carbon release and biodegradability study of the denitrifying media
The elemental compositions of the WOSS were analyzed by CHNS elemental analyzer and are given in Table 1. The WOSS were subjected to rigorous leaching study to figure out leachable organic carbons. Approximately equal volume of WOSS (1 g of SB and GS and 5 g of CS and Mix) were taken in 250 mL Erlenmeyer flasks containing 50 mL of distilled water agitated at 200 rpm for 7 days at room temperature. The supernatant was then analyzed for total organic carbon after filtration with 0.45 μm filter paper. Samples were taken every 7 days for TOC measurements, and 50 mL of fresh water was added each time. The organic carbon release rate for a period of 30 days was calculated as per eqn (1).| CR = (C × V)/(W × t) | (1) |
| DR (%) = (W0 − Wt)/(W0 × 100) | (2) |
| Properties and compositions | SB | GS | CS |
|---|---|---|---|
| Porosity (%) | 82 ± 3 | 76 ± 2 | 69 ± 2 |
| Specific yield (%) | 57 ± 1 | 54 ± 2 | 61 ± 2 |
| Specific retention (%) | 25 ± 0.5 | 22 ± 1 | 6 ± 1 |
| C (%) | 44.56 | 48.73 | 49.83 |
| H (%) | 5.719 | 5.645 | 5.660 |
| N (%) | 0.13 | 0.51 | 0.08 |
| TOC (%) | 58.55 | 69.39 | 68.26 |
| SCOD (mg g−1) | 496.75 | 319.25 | 106.75 |
| Properties and compositions | Dolochar |
|---|---|
| SiO2 (%) | 42.39 ± 1.61 |
| CaO (%) | 17.76 ± 0.74 |
| Al2O3 (%) | 15.63 ± 1.67 |
| Fe2O3 (%) | 14.17 ± 1.03 |
| MgO (%) | 5.44 ± 0.56 |
| BET surface area (m2 g−1) | 63.433 |
| Langmuir surface area (m2 g−1) | 97.564 |
| D–R micro-pore surface area (m2 g−1) | 93.698 |
2.5. Real domestic wastewater
Real domestic wastewater samples were collected from the wastewater treatment plant (WTP), Keshura, Bhubaneswar, Odisha. The WTP receives domestic wastewater discharge of around 300 families residing in the residential complex. The characteristics of the domestic wastewater before and after treatment are given in Table 2. The wastewater was further spiked with calculated amount of anhydrous NH4Cl, NaNO3, NaNO2, KH2PO4 etc., to get different desired concentrations of ammonium-nitrogen, nitrate-nitrogen, nitrite-nitrogen and phosphate-phosphorous, respectively whenever required for experiments. The pH of the nutrient-spiked domestic wastewater was not controlled in this study. To enhance the metabolic activity of the concerned microbes, micro and macro nutrient were added to the domestic wastewater. All the chemicals used in this study were of analytical grades and procured from Merck, Mumbai.| Compositions | Before treatment | After treatment |
|---|---|---|
| pH | 6.77 ± 0.5 | 7.54 ± 0.2 |
| DO (mg L−1) | 1.19 ± 0.2 | 0.99 ± 0.1 |
| COD (mg L−1) | 217 ± 10 | 61.89 ± 2 |
| BOD5 (mg L−1) | 98 ± 3 | 43 ± 1 |
| TDS (mg L−1) | 292 ± 5 | 107 ± 2 |
| NH4+-N (mg L−1) | 47.38 ± 5 | 9.8 ± 0.5 |
| NO3−-N (mg L−1) | 7.1 ± 0.3 | 0.92 ± 0.2 |
| NO2−-N (mg L−1) | 0.5 ± 0.3 | 0.15 ± 0.05 |
| Total phosphate (mg L−1) | 10.92 ± 1 | 0.87 ± 0.2 |
2.6. Seeding, acclimatization and experimental procedure
During the start-up period, the integrated system was seeded with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of domestic wastewater and activated sludge. The inoculated sludge was obtained from a laboratory scale UASB reactor with the concentration of 4218 mg L−1 of MLSS (mixed liquor suspended solid) and 2198 mg L−1 of MLVSS (mixed liquor volatile suspended solid). After inoculation, the domestic wastewater with added micro and macro nutrient was replaced after every 24 h in a batch mode of operation. The batch mode continued for a period of 2 weeks to offer initial accumulation and colonization of the microorganisms. Then the system was subjected to a continuous mode of operation with a hydraulic loading rate of 80 L m−2 day−1 which corresponded to a hydraulic retention time (HRT) of 22.5 h for the trickling bio-filter (considering 5 stages) and denitrifying bio-reactor as well. Though, it was hard to provide the HRT of each stage in the multi-stage trickling bio-filter, roughly 4.5 h of HRT was calculated for the individual stages. During this period, domestic wastewater spiked with ammonium-nitrogen and phosphate-phosphorous was circulated in the system. Gradual increase in nutrient loading rate was encouraged after every fixed interval time of 7 days. The purpose was to acclimatize the biomass by exposing them to increased dose of nutrient. The continuous operation continued till the system reached the steady-state condition after passing through a pseudo steady-state condition. The pseudo steady-state condition was supposed to be achieved when the variation in effluent COD concentration was found to be insignificant, whereas stable removal rate of nutrient marked the onset of steady state.
1 volume ratio of domestic wastewater and activated sludge. The inoculated sludge was obtained from a laboratory scale UASB reactor with the concentration of 4218 mg L−1 of MLSS (mixed liquor suspended solid) and 2198 mg L−1 of MLVSS (mixed liquor volatile suspended solid). After inoculation, the domestic wastewater with added micro and macro nutrient was replaced after every 24 h in a batch mode of operation. The batch mode continued for a period of 2 weeks to offer initial accumulation and colonization of the microorganisms. Then the system was subjected to a continuous mode of operation with a hydraulic loading rate of 80 L m−2 day−1 which corresponded to a hydraulic retention time (HRT) of 22.5 h for the trickling bio-filter (considering 5 stages) and denitrifying bio-reactor as well. Though, it was hard to provide the HRT of each stage in the multi-stage trickling bio-filter, roughly 4.5 h of HRT was calculated for the individual stages. During this period, domestic wastewater spiked with ammonium-nitrogen and phosphate-phosphorous was circulated in the system. Gradual increase in nutrient loading rate was encouraged after every fixed interval time of 7 days. The purpose was to acclimatize the biomass by exposing them to increased dose of nutrient. The continuous operation continued till the system reached the steady-state condition after passing through a pseudo steady-state condition. The pseudo steady-state condition was supposed to be achieved when the variation in effluent COD concentration was found to be insignificant, whereas stable removal rate of nutrient marked the onset of steady state.
The removal efficiency (RE) was calculated based on the aqueous concentrations of influent and effluent as per the following formula:31
| RE (%) = (C0 − Ce)/(C0 × 100) | (3) |
2.7. Analytical methods
Filtration of the samples was done using 0.45 μm filter paper, prior to analysis. Phosphate concentration in aqueous solutions was determined by the vanado molybdo phosphoric acid method. 1 mL of vanadate-molybdate reagent, 0.5 mL of distilled water and 3.5 mL of filtered sample were mixed and the solution was analyzed after 10 min at the detection wavelength of 470 nm. Nitrate-nitrogen concentration was determined by the sodium salicylate method.32 10 mL of filtered sample with 1 mL freshly prepared sodium salicylate was evaporated in an evaporating dish, cooled, 1 mL concentrated H2SO4 was added for dehumidification of the entire residue. The volume was adjusted to 50 mL with 7 mL 10 molar NaOH and distilled water. The absorbance was measured at the detection wavelength of 410 nm after 10 min of incubation against a blank prepared in the same way. Nitrite-nitrogen was measured by sulfanilic acid method.32 To 5 mL of filtered sample 0.1 mL of sulfanilic acid was added and after 5 min 0.1 mL of α-naphtylamine was added and mixed. After 40 min of incubation, absorbance was measured at 520 nm against the blank. Ammonium-nitrogen was detected by using Nessler reagent.32 To 5 mL of filtered sample 0.1 mL of Seignettsalt was added and after 5 min 0.1 mL of Nessler reagent was added and mixed. After 10 min of incubation, absorbance was measured at 425 nm against the blank. The absorbance was measured in a UV/VIS spectrophotometer (Perkin-Elmer Lambd-25) for all the above cases. The results were cross-verified by ion selective electrode (ISE) methods for nitrate, nitrite, and ammonium nitrogen using the respective probes of Orion 5-star multi-parameter meter. Chemical oxygen demand (COD), dissolved oxygen (DO) and pH were determined respectively, colorimetric method (5220-D), Orion 5-star DO probe and HACH digital pH meter as per the procedure mentioned in the standard methods for the examination of water and wastewater.33 The XRD analysis was done in the 2θ range of 15–70° in Bruker D8 DISCOVER diffractometer with Cu Kα radiation operated at 40 kV, 40 mA with a scan speed of 3° min−1 KBr disks containing approximately 2 mg dolochar and 200 mg KBr were prepared and the FTIR spectra were obtained from Bruker ALPHA-FTIR spectrophotometer by averaging 64 scans from 500 to 4000 cm−1, at a resolution of 4 cm−1. The nutrient saturated dolochar was subjected to ‘thin layer in funnel’ analytical test as described in Section 2.7 to figure out the nutrient leaching ability of the saturated dolochar. Elemental distribution of dolochar before and after treatment was monitored using a field emission scanning electron microscope (FESEM, ZEISS SUPRA 55), equipped with EDS at an accelerating voltage of 15 kV by sprinkling adsorbents onto the carbon tape mounted on the SEM stub. The FTIR spectra of WOSS (2 mg sample in 200 mg KBr) were recorded on Bruker ALPHA-FTIR Spectrophotometer by setting the scanning range of 500–4000 cm−1, resolution of 4 cm−1 and a scanning rate of 64. Elemental composition of WOSS before and after denitrification process was analyzed by an elemental analyzer (Elementar-Vario MICROCUBE) operated at oxidation tube temperature 1150 °C, reduction tube temperature 850 °C, detector temperature (TCD) 59 °C, helium flow rate 200 mL min−1 and oxygen flow rate 4 m min−1. Total organic carbon (TOC) released from WOSS was determined by means of a high-temperature aqueous TOC analyzer (Elementar-VarioTOC CUBE) operated at 850 °C temperature, 1000 mbar pressure, and 180 mL min−1 gas flow.2.8. Nutrient leaching study
To establish the applicability of the spent adsorbent as a nutrient supplier, ‘thin layer in funnel’ analytical test was performed by following the slightly modified procedure as described by Bhardwaj et al.34 In this study, approximately 5 g of nutrient loaded dolochar was deposited on filter paper already kept inside a Buchner funnel having diameter 5 cm. The funnel was intermittently flooded after every 24 h with 100 mL of distilled water by adding 20 mL in every 30 min and the filtrate collected was analyzed for released nutrient.93. Results and discussions
3.1. Characterization of filter bed materials before and after treatment process
Dolochar, a waste material generated from sponge iron industry, consists of devolatilized dolomite and char, was used as the filter media in the trickling bio-filter. The presence of fused carbon, lime materials, and metallic iron as three distinct phases play a pivotal role in physicochemical nutrient removal from wastewater. The detailed characterization of dolochar was done by various analytical techniques like XRD, FTIR, EDS, BET, etc. and the results are depicted in one of our previous studies, Rout et al. 2015.35 The data in Table 3 revealed the occurrence of Si, Ca, Al, Fe and Mg-oxides as the major chemical constituents. It is also noticed from the table that the Langmuir, BET and micro pore surface area of the dolochar sample are 97.56, 63.43 and 93.69 m2 g−1 and respectively. These textural parameters and chemical compositions of dolochar signify that the sample is prone to physicochemical adsorption. Nutrient entities, phosphate in particular, gets precipitated in the presence of Mg and Ca-oxides and reacts with Fe and Al-oxides by ligand exchange forming inner-sphere complexes. The innate porosity of dolochar is believed to play a vital role in removing other nutrient species via physicochemical means.| Characteristics | SB | GS | CS | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Weight (g) | 2.5 ± 0.5 | 1.87 ± 0.3 | 2.5 ± 0.5 | 2.03 ± 0.1 | 10 ± 0.5 | 9.3 ± 0.2 |
| Degradation rate (%) | 25.2 ± 0.2 | 18.8 ± 0.1 | 6.94 ± 0.1 | |||
| Porosity (%) | 82 ± 3 | 36.7 ± 1 | 76 ± 2 | 38.3 ± 1 | 69 ± 2 | 40.5 ± 1 |
| Change in porosity (%) | 55.19 ± 0.12 | 49.6 ± 0.5 | 41.3 ± 0.2 | |||
| C content (%) | 44.56 | 46.55 | 48.73 | 49.13 | 48.94 | 49.83 |
| N content (%) | 0.13 | 1.36 | 0.49 | 0.51 | 0.08 | 0.1 |
| C/N | 342.76 | 34.22 | 99.44 | 96.33 | 611.75 | 498.3 |
| Major FTIR peaks (cm−1) | 3406 | 3386 | 3410 | 3405 | 3407 | 3410 |
| 2918 | 2922 | 2921 | 2923 | 2923 | 2921 | |
| 1732 | 1729 | 1737 | 1737 | 1734 | 1736 | |
| 1248 | 1246 | 1247 | 1265 | 1248 | 1247 | |
| 1160 | 1160 | 1164 | — | 1164 | 1164 | |
| 1041 | 1045 | 1045 | 1034 | 1046 | 1045 | |
| 607 | 608 | 607 | 608 | 608 | 607 | |
The process of nutrient removal in the trickling bio-filter was illustrated with the help of information obtained by undertaking analytical techniques like, XRD, FTIR and EDS. From XRD spectra, it was observed that the peaks matching to ferrite phase in the 2θ range of 30–40 in the spectrum of native dolochar sample are either diminished in intensity or disappeared from the spectrum of dolochar after subjected to treatment process. Similarly, the appearance of new peaks matching to aluminum phosphide, iron phosphate and magnesium phosphate were observed only in the spectrum of dolochar after treatment. The emergence of the mentioned peaks may be attributed to the phosphate adsorption whereas the loss of ferrite peak may be correlated to the involvement of ferrite phase in phosphate adsorption process. The FTIR spectra of dolochar before and after treatment process revealed that the entire spectrum was more or less similar in terms of band position and frequency. However, the appearance of a new peak at 874 cm−1 in the spectrum of dolochar after treatment indicated the participation of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O entities in the adsorption process, since the same peak was absent from the spectrum of dolochar before treatment. Nonetheless, to avoid confusion regarding the presence of the peak at 870 cm−1 in native dolochar and to get a more clear picture regarding phosphate adsorption, EDS analysis of all the samples were carried out. The EDS spectra of dolochar before and after treatment process are shown in the Fig. 2. Fig. 2a displays the EDS spectra of the entire electron image of the dolochar sample before subjected to the treatment process. The presence of C, O, Ca, Al, Si, Fe, Mg etc. as primary elements were observed from the figure. Fig. 2b and c display the EDS spectra of two different point specific electron images of dolochar sample after going through the treatment process. The surfacing of a peak corresponding to nitrogen in Fig. 2b and emergence of a phosphorous peak in the Fig. 2c authenticate the successful adsorption of both nitrogen and phosphorous entities onto dolochar and thereby, validating the nutrient removal in the trickling bio-filter through physicochemical mode, where dolochar played the imperative role. Since the EDS images provided the direct visual evidence of phosphate adsorption, the spectral images of XRD and FTIR were not included in this manuscript.
O entities in the adsorption process, since the same peak was absent from the spectrum of dolochar before treatment. Nonetheless, to avoid confusion regarding the presence of the peak at 870 cm−1 in native dolochar and to get a more clear picture regarding phosphate adsorption, EDS analysis of all the samples were carried out. The EDS spectra of dolochar before and after treatment process are shown in the Fig. 2. Fig. 2a displays the EDS spectra of the entire electron image of the dolochar sample before subjected to the treatment process. The presence of C, O, Ca, Al, Si, Fe, Mg etc. as primary elements were observed from the figure. Fig. 2b and c display the EDS spectra of two different point specific electron images of dolochar sample after going through the treatment process. The surfacing of a peak corresponding to nitrogen in Fig. 2b and emergence of a phosphorous peak in the Fig. 2c authenticate the successful adsorption of both nitrogen and phosphorous entities onto dolochar and thereby, validating the nutrient removal in the trickling bio-filter through physicochemical mode, where dolochar played the imperative role. Since the EDS images provided the direct visual evidence of phosphate adsorption, the spectral images of XRD and FTIR were not included in this manuscript.
 | ||
| Fig. 2 (a) EDS images of dolochar before treatment, (b) EDS images of dolochar demonstrating nitrogen removal, (c) EDS images of dolochar demonstrating phosphorous removal. | ||
3.2. Characterization of denitrifying media before and after treatment process
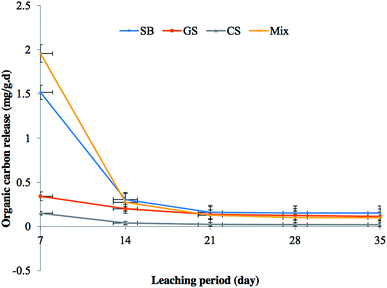
The leaching study was carried out to quantify the amount of carbon released by the fresh WOSS under consideration. Fig. 3 shows the organic carbon release rate of the three WOSS and the Mix one in distilled water. On the 7th day, the organic carbon release rate of all the flasks under consideration was very high. The high carbon release rate of 1.96 and 1.5 mg g−1 d−1 was reported for Mix and SB, respectively as compared to GS (0.34 mg g−1 d−1) and CS (0.15 mg g−1 d−1) when there were no microorganisms present. The presence of labile and readily soluble carbon is responsible for the high carbon release in case of SB and Mix since SB is also a part of Mix. The release rate dropped down to a significantly low level on the 14th day and slowly stabilized from then onwards. During the stable period, the organic carbon release in all the four flasks varied little and the organic carbon release rate followed the order SB > GS > Mix > CS. The results as depicted in Fig. 3 demonstrate that the organic carbon release rate of individual WOSS and the Mix was slow and stable and hence they could offer sufficient organic carbon continuously to the microorganisms for the denitrification process. Similar type of observations have also been reported in earlier publications.36The biodegradation of the WOSS was first inspected visually. There was a noticeable color change in case of all the WOSS and the WOSS after biodegradation appeared to be darker than the fresh WOSS. Some SB and GS were reduced in size to strands or fibers from the original size due to biodegradation. There was almost no size reduction observed in the case of CS. Physically the degradation was assessed by measuring degradation rate as well as changes in the media porosity as per the values mentioned in Table 3. The degradation rate for the considered WOSS follows the order as SB > GS > CS with the respective values of 25.2 ± 0.2, 13.57 ± 0.3, and 6.94 ± 0.1%. Based on these results, it can be stated that CS would last longer than SB. The loss of mass of WOSS may be attributed to the loss of the water soluble component, a significant portion of the cellulose and hemicellulose by the end of the denitrification experiment. The change in porosity percentage for SB, GS and CS were reported to be 55.19 ± 0.12, 49.6 ± 0.5 and 41.3 ± 0.2%, respectively (Table 3), which was caused by the swelling as well as biodegradation of the media and accumulation of microbial biomass. SB exhibited more swelling than GS followed by CS. Additionally, the chemical basis of biodegradability analysis of the media was done by taking C/N ratio into account. The composition of carbon and nitrogen of all the WOSS before and after utilization were measured and the values are noted in Table 3. An increase in the mass of carbon was observed for all the WOSS under consideration, which might be due to the contribution of microbial biomass carbon that got immobilized in the micro pores of the media. Nitrogen fortification was also noticed in case of all the WOSS, hence, the media C/N ratio decreased. As per literature, low C/N ratios favor biodegradation. SB had the lowest C/N after utilization followed by GS, and CS. So results from all the means of measurement of the biodegradability e.g., visual, physical and chemical are in agreement with each other.
The determination of changes in chemical structure of WOSS before and after utilization was done by FTIR spectroscopy. The related spectra are displayed in Fig. 4a–c and the comparison of peaks before and after biodegradation were presented in Table 3. The results demonstrate that the position of featured peaks in the spectra of utilized WOSS had not altered a lot as compared to raw ones, but the change in peak intensities was observed. For example, a broad peak in 3400 cm−1 region due to the stretching of the OH groups decreased sharply in almost all the spectra of WOSS after utilization. Similarly, peaks around 2920 cm−1 (stretching of CH groups) and around 1735 cm−1 (due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups) decreased slightly in case of spectra of utilized SB, AS, GS and CS.37 The relative lower intensity of peaks as observed in the spectra of spent WOSS as compared to raw WOSS might be due to the biodegradation of WOSS in the process of denitrification.31 The overall FTIR analysis indicates that the biodegradation does not significantly change the chemical structure of WOSS. Similar phenomena have been also reported by earlier researchers.7
O groups) decreased slightly in case of spectra of utilized SB, AS, GS and CS.37 The relative lower intensity of peaks as observed in the spectra of spent WOSS as compared to raw WOSS might be due to the biodegradation of WOSS in the process of denitrification.31 The overall FTIR analysis indicates that the biodegradation does not significantly change the chemical structure of WOSS. Similar phenomena have been also reported by earlier researchers.7
 | ||
| Fig. 4 (a) FTIR images of SB before and after treatment (b) FTIR images of GS before and after treatment (c) FTIR images of CS before and after treatment. | ||
3.3. Performance of integrated system under steady state condition
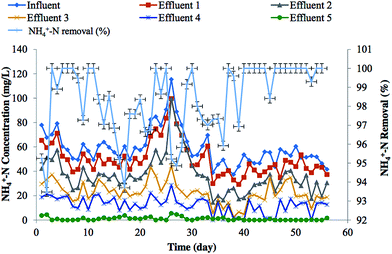 | ||
| Fig. 6 Variations in the concentration of NH4+-N in the influent, effluents of different stages and removal efficiency in trickling bio-filter. Standard error bars shown wherever applicable. | ||
 | ||
| Fig. 7 Variations in the concentration of NO3−-N in the influent and effluents of different stages of trickling bio-filter. Standard error bars shown wherever applicable. | ||
 | ||
| Fig. 9 Variations in the concentration of NO3−-N in the influent, effluents and removal efficiency in denitrifying bio-reactor. Standard error bars shown wherever applicable. | ||
Fig. 10 represents the release of SCOD from the denitrifying bio-reactor effluent during denitrification process. The organic carbon concentration in the effluent was initially in the range of 850 mg L−1 and then declined during the experiment to the range of 180 mg L−1 on the 10th day of operation. This rapid release of organic carbon was due to wash out of the soluble carbon in excess of the requirement by the denitrifying biomass. Also, some mass of organic carbon was contributed by the activated sludge. With the growth of the microbial biomass, the organic carbon got consumed and subsequently, effluent SCOD concentration decreased. In the meantime, the microbes get accumulated and immobilized on the WOSS to form the bio-film and start to degrade the solid organic carbon enzymatically to get the soluble carbon for their growth and vitality.42 The release pattern implies that the carbon release from WOSS does not occur steadily. So the amount to be added and frequency of addition need to be optimized for maximum benefit.43
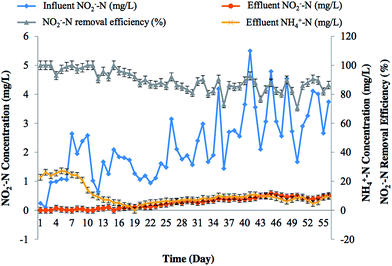
The Fig. 11 demonstrates that the NO2−-N removal in denitrifying bio-reactor followed almost similar trend than that of NO3−-N removal. More concentration of NO2−-N in the effluent with the passage of time may be explained on the basis of washout of SCOD and loosely attached or unattached biomass with the continuous flow of wastewater or due to the blockage of denitrifying bio-reactor by falling-off bio-films that ultimately results in decreased denitrification. Sometimes increasing NO2−-N accumulation and subsequent inhibition in NO2−-N reduction rates are due to the temporary repression of nitrite reductase from over-competition with nitrate reductase for electrons.44
High NH4+-N accumulation was observed during start up period of the denitrification experiment as shown in Fig. 11. Elevated NH4+-N accumulation during the initial operating period may be attributed to the process of dissimilatory nitrate reduction to ammonia (DNRA).45 The process is carried out by fermentative bacteria when the reduction of organic matter is not possible. High C/N ratios are thought to favor the DNRA process.46,47 In this way, the finding of this study is in agreement with the earlier findings. The reduction of the NH4+-N concentration in due course of time may be due to its assimilation into organic matter by microbial biomass. Sometimes sudden increased in effluent concentration NH4+-N was also observed, which may arise from the death and decomposition of the microbe inside the bio-reactor.
3.4. Reutilization of spent dolochar and partially degraded WOSS
Fig. 12 represents the results of funnel analytical test which figures out that, the rate of phosphate and nitrate release by the nutrient loaded dolochar to be 24–26% and 18–20% of the adsorbed amount, respectively, after 1st cycle. The respective values decreased down to 15–17% and 12–14%, after the 3rd cycle. The readily soluble form of the nutrient that covers the dolochar surface and that has been unbound or weakly bound to the surface are the causes for the rapid release of nutrient in the initial stages. However, nutrient release rate stabilizes progressively from the 3rd cycle onwards, and respective phosphate and nitrate release rates of about 4–6% and 6–8%, of adsorbed amount are continued to appear in the filtrate even after 10th cycle. So nutrient release pattern of dolochar follows a two-stage mechanism of fast release followed by slow release.34,48 Therefore, it can be stated that dolochar has a propensity to hold the nutrient and release them slowly. Thus, it can be used as a slow release nutrient supplier and we have used the same as a source of nutrient to grow ornamental plants in our lab (Fig. 1b). Similarly, partially degraded WOSS can be subjected to composting and can be used as an organic fertilizer or as a bed material for vermi-filter. In our lab, we have composted the spent WOSS (Fig. 1b) and we are using the same in the vermi-filters that are being used for treatment of various kinds of wastewater in our lab.4. Conclusions
The designed integrated system consisting of a multi-stage trickling bio-filter with drop aeration facility and a denitrifying bioreactor, displayed high removal efficiency of nutrients from domestic wastewater. Trickling bio-filter packed with dolochar enhanced PO43−-P removal remarkably due to its innate porosity and presence of reactive Fe, Al, Ca and Mg-oxides as its chemical constituent. Drop aeration mediated nitrification resulted in DO concentration in the range of 2.5–5 mg L−1 in each stage of bio-filter unit which ensured sufficient oxygenation to keep the effluent NH4+-N concentration below 5.5 mg L−1. Complete nitrogen removal by denitrification was realized in the denitrifying bio-reactor, which relied on WOSS as a suitable organic carbon source for the denitrifying biomass, thereby making the system cost effective. In addition, thin layer funnel analytical test results confirmed the possible utility of spent dolochar as a nutrient supplier whereas the spent WOSS was used as a bed material in vermi-filters. Therefore, the designed integrated system having a lot of added advantages can be adjudged as a promising alternative for nutrient removal from rural domestic wastewater in developing countries.Acknowledgements
The authors would like to thank the editors and anonymous reviewers for reviewing the manuscript and for their expedient commentaries. The authors are thankful to the School of Infrastructure, Indian Institute of Technology Bhubaneswar, India, for providing facilities to carry out the research work in the concerned area.References
- Y. Wu, W. Zhu and X. Lu, Ecol. Eng., 2013, 61, 166–173 CrossRef.
- M. Ockenden, J. Quinton, N. Favaretto, C. Deasy and B. Surridge, Environ. Sci.: Processes Impacts, 2014, 16, 1637–1645 CAS.
- S. Hussain, H. A. Aziz, M. H. Isa, A. Ahmad, J. Van Leeuwen, L. Zou, S. Beecham and M. Umar, Desalination, 2011, 271, 265–272 CrossRef CAS.
- L. Wang, F. Guo, Z. Zheng, X. Luo and J. Zhang, Bioresour. Technol., 2011, 102, 9462–9470 CrossRef CAS PubMed.
- P. R. Rout, P. Bhunia and R. R. Dash, J. Taiwan Inst. Chem. Eng., 2015, 46, 98–108 CrossRef CAS.
- S. Yang, W. Guo, Y. Chen, X. Zhou, H. Zheng, X. Feng, R. Yin and N. Ren, RSC Adv., 2014, 4, 52892–52897 RSC.
- J. Mai, X. Chai, L. Su, Q. Li and X. Zhao, RSC Adv., 2016, 6, 6528–6539 RSC.
- M. Yuan, W. W. Carmichael and E. D. Hilborn, Toxicon, 2006, 48, 627–640 CrossRef CAS PubMed.
- P. R. Rout, R. R. Dash and P. Bhunia, Process Saf. Environ. Prot., 2016, 100, 91–107 CrossRef CAS.
- W. Luo, C. Yang, H. He, G. Zeng, S. Yan and Y. Cheng, Ecol. Eng., 2014, 64, 415–423 CrossRef.
- A. M. Kadam, P. D. Nemade, G. Oza and H. S. Shankar, Ecol. Eng., 2009, 35, 1051–1061 CrossRef.
- M. G. Healy, M. Rodgers and J. Mulqueen, Bioresour. Technol., 2007, 98, 2268–2281 CrossRef CAS PubMed.
- M. A. Massoud, A. Tarhini and J. A. Nasr, J. Environ. Manage., 2009, 90, 652–659 CrossRef PubMed.
- Y. Chun, H. Zhan-Bo, K. Hai-Nan, W. Xin-Ze and H. Sheng-Bing, Pedosphere, 2008, 18, 401–408 CrossRef.
- Q. Zhou, S. He, X. He, X. Huang, B. Picot, X. Li and G. Chen, Water Sci. Technol.: Water Supply, 2006, 6, 43–50 CrossRef CAS.
- L. Zhang, S. Hong, C. Wen, X. Mao, A. Liu and F. Gan, Environ. Eng. Sci., 2009, 26, 775–782 CrossRef CAS.
- X. Ren, H. Shon, N. Jang, Y. G. Lee, M. Bae, J. Lee, K. Cho and I. S. Kim, Water Res., 2010, 44, 751–760 CrossRef CAS PubMed.
- T. Ichinari, A. Ohtsubo, T. Ozawa, K. Hasegawa, K. Teduka, T. Oguchi and Y. Kiso, Process Biochem., 2008, 43, 722–728 CrossRef CAS.
- J. Ham, C. Yoon, J. Jeon and H. Kim, Water Sci. Technol., 2007, 55, 503–511 CrossRef CAS PubMed.
- J. Vymazal, Sci. Total Environ., 2007, 380, 48–65 CrossRef CAS PubMed.
- J. Vymazal and L. Kröpfelová, Ecol. Eng., 2011, 37, 90–98 CrossRef.
- J. Vymazal and L. Kröpfelová, Wastewater treatment in constructed wetlands with horizontal sub-surface flow, Springer Science & Business Media, 2008 Search PubMed.
- A. R. Jantrania and M. A. Gross, Advanced onsite wastewater systems technologies, CRC Press, 2006 Search PubMed.
- P. R. Rout, P. Bhunia and R. R. Dash, J. Environ. Chem. Eng., 2014, 2, 1331–1342 CrossRef CAS.
- X. Zheng, Y. Su, Y. Chen, Y. Wei, M. Li and H. Huang, RSC Adv., 2014, 4, 45953–45959 RSC.
- P. Chowdhury, T. Viraraghavan and A. Srinivasan, Bioresour. Technol., 2010, 101, 439–449 CrossRef CAS PubMed.
- L. Christianson, M. Helmers, A. Bhandari and T. Moorman, Ecol. Eng., 2013, 52, 298–307 CrossRef.
- Y. Yang, Y. Inamori, H. Ojima, H. Machii and Y. Shimizu, Water Res., 2005, 39, 4859–4868 CrossRef CAS PubMed.
- O. Gibert, S. Pomierny, I. Rowe and R. M. Kalin, Bioresour. Technol., 2008, 99, 7587–7596 CrossRef CAS PubMed.
- Y. Xu, T.-L. Qiu, M.-L. Han, J. Li and X.-M. Wang, Procedia Environ. Sci., 2011, 10, 72–77 CrossRef CAS.
- S.-M. Zhu, Y.-L. Deng, Y.-J. Ruan, X.-S. Guo, M.-M. Shi and J.-Z. Shen, Bioresour. Technol., 2015, 192, 603–610 CrossRef CAS PubMed.
- A. Bartošová, A. Michalíková, M. Sirotiak and M. Soldán, Res. Pap.–Fac. Mater. Sci. Technol., Slovak Univ. Technol., 2012, 20, 8–19 Search PubMed.
- A. Apha, Standard methods for the examination of water and wastewater, 2005, vol. 17, pp. 1.5–3.12 Search PubMed.
- D. Bhardwaj, M. Sharma, P. Sharma and R. Tomar, J. Hazard. Mater., 2012, 227, 292–300 CrossRef PubMed.
- P. R. Rout, P. Bhunia and R. R. Dash, J. Taiwan Inst. Chem. Eng., 2015, 46, 98–108 CrossRef CAS.
- J. Zhang, C. Feng, S. Hong, H. Hao and Y. Yang, Water Sci. Technol., 2012, 65, 1696 CrossRef CAS PubMed.
- Z. Shen and J. Wang, Bioresour. Technol., 2011, 102, 8835–8838 CrossRef CAS PubMed.
- T. Nur, M. A. H. Johir, P. Loganathan, T. Nguyen, S. Vigneswaran and J. Kandasamy, J. Ind. Eng. Chem., 2014, 20, 1301–1307 CrossRef.
- P. Tomar and S. Suthar, Desalination, 2011, 282, 95–103 CrossRef CAS.
- E. M. Ruane, P. N. Murphy, M. G. Healy, P. French and M. Rodgers, Water Res., 2011, 45, 6668–6676 CrossRef CAS PubMed.
- J. Zou, X. Guo, Y. Han, J. Liu and H. Liang, Water, Air, Soil Pollut., 2012, 223, 889–900 CrossRef CAS.
- R. Zhang, Y. Zhang, F. Lv, H. Wang and S. Tu, Desalin. Water Treat., 2015, 1–8 Search PubMed.
- J. Park, R. Craggs and J. Sukias, Bioresour. Technol., 2009, 100, 3175–3179 CrossRef CAS PubMed.
- Z. Hu, J. Zhang, S. Li, J. Wang and T. Zhang, Bioresour. Technol., 2011, 102, 5486–5491 CrossRef CAS PubMed.
- H. Hamlin, J. Michaels, C. Beaulaton, W. Graham, W. Dutt, P. Steinbach, T. Losordo, K. Schrader and K. Main, Aquacultural Engineering, 2008, 38, 79–92 CrossRef.
- J. Van Rijn, Y. Tal and H. J. Schreier, Aquacultural Engineering, 2006, 34, 364–376 CrossRef.
- J. M. Tiedje, Biology of anaerobic microorganisms, 1988, vol. 717, pp. 179–244 Search PubMed.
- A. K. Bansiwal, S. S. Rayalu, N. K. Labhasetwar, A. A. Juwarkar and S. Devotta, J. Agric. Food Chem., 2006, 54, 4773–4779 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |