Facile synthesis of core–shell magnetic-fluorescent nanoparticles for cell imaging†
Abstract
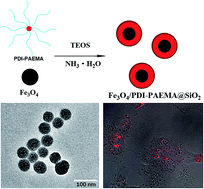
In this work, we present a novel type of magnetic-fluorescent bifunctional nanoparticle (NP). Fe3O4 nanocrystals and cationic fluorescent star polymer perylene diimide–poly(2-aminoethyl methacrylate) (PDI–PAEMA) were simultaneously encapsulated into a silica matrix by a one-pot method. The morphology and fluorescence properties of Fe3O4/PDI–PAEMA@SiO2 core–shell NPs were investigated by ultraviolet-visible (UV-vis) spectrometry, fluorescence spectrometry and high resolution transmission electron microscopy (HRTEM). The analysis of in vitro intracellular uptake and cell viability revealed that the bifunctional NPs possessed favourable biocompatibility. Taken together, the Fe3O4/PDI–PAEMA@SiO2 NPs could be a promising candidate for bioimaging due to their stable red emission and favourable biocompatibility.

 Please wait while we load your content...
Please wait while we load your content...