Photochemically assisted one-pot synthesis of PMMA embedded silver nanoparticles: antibacterial efficacy and water treatment†
Shubhangi Borsea,
Mayur Temgireb,
Ayesha Khana and
Satyawati Joshi*a
aDepartment of Chemistry, Savitribai Phule Pune University, (Formerly University of Pune) Ganeshkhind, Pune 411007, India. E-mail: ssjoshi@chem.unipune.ac.in; Fax: +91 020 25691728; Tel: +91 20 25601394 ext. 573, 569,532
bDepartment of Chemical Engineering, Indian Institute of Technology Bombay, Powai, Mumbai 400076, India
First published on 1st June 2016
Abstract
One-pot synthesis of polymer embedded silver nanoparticles (AgNPs) by the UV irradiation method was performed and thoroughly characterized. Electron microscopy analysis revealed that AgNPs (17.30 nm) were embedded into the PMMA matrix. High-resolution transmission electron microscopy (HRTEM) study indicates that the silver–poly(methyl methacrylate) (Ag–PMMA) nanocomposite exhibited internal highly ordered lattice fringes of the Ag (111) lattice plane. The Ag–PMMA nanocomposite had a lower tendency to agglomerate than borohydride reduced AgNPs as evaluated by stability experiments. The nanocomposite shows a zeta potential of −63.9 mV confirming the high stability of the nanocomposite. Organic reagent tests reveal that the synthesized nanocomposite contains a greater amount of Ag0-state particles and a lesser amount of Ag+ ions. Fourier transform infrared spectroscopy (FTIR) studies evidenced that the carbonyl group of PMMA binds to AgNPs. The nanocomposite exhibited excellent antibacterial performance against Gram negative Escherichia coli, Pseudomonas aeruginosa and Gram positive, Staphylococcus aureus. Moreover, the possible mechanism for the antibacterial activity of the Ag–PMMA nanocomposite with E. coli bacteria was discussed. In addition, the Ag–PMMA nanocomposite solution was loaded onto a membrane (the ‘treated membrane’) for water treatment applications and characterized by spectroscopy techniques. Sludge water was passed through the treated membrane and the effluent water was analyzed for viable bacteria. Deactivation of bacteria by percolation through the treated membrane occurred. Consequently, the filter effluent contains dead bacteria, which indicates that the treated membrane exhibits antibacterial properties. Interestingly, microwave plasma-atomic emission spectrometry (MP-AES) analysis estimated that silver loss in the treated membrane was less than 0.1 ppm.
Introduction
Water will be the number one global risk of highest impact in the next ten years, according to the 2015 Global Risk Report. Analytical studies have projected our global demand for water will exceed available supply by 40% in 2030, and by 2050, an estimated $63 trillion of global Gross Domestic Product (GDP) will be put at risk under ‘business as usual’ water management practices and productivity.1 Natural waters are frequently contaminated mostly through discharge of sewage and industrial waste water, which encompass toxic or carcinogenic impurities causing numerous public health complications. Generally chlorination, ozonation and UV-treatment methods are used for water disinfection.2–6The basic aim of water treatment is to remove undesired constituents from water.6 The use of membrane technology in water treatment has gained impetus for treating microorganisms, particulates and organic materials that contaminate water. Membrane based technology is a promising disinfection method in which microorganisms are retained without any harmful chemical treatment. Membrane technology is well established for water treatment because it is a reliable and largely automated process. Membranes deliver a physical barrier for such constituents based on their size, permitting the use of unconventional water sources.7 However, biofouling is a serious problem that occurs during the membrane filtration process due to deposition of microbial cells or other organic matter present in the feed stream.8 Nanotechnology has great potential in advancing water and waste water treatment to improve treatment effectiveness and to increase water supply through the safe use of unconventional water sources.6,7 Incorporation of nanomaterials into membranes offers a great prospect to improve membrane permeability, fouling resistance, and mechanical and thermal stability, in addition to rendering new functions for contaminant degradation and self-cleaning.9,10 Particularly, biocidal silver nanoparticles (AgNPs) are effective disinfectants and work for a wide spectrum of bacteria and viruses.11–13 AgNPs have been used previously in water filtration applications, as they prevent bacterial fouling of membrane filters. Compared to AgNPs, immobilized AgNPs are physico-chemically more stable as they are less prone to aggregation and oxidation when exposed to aqueous media.14 Beacuse of this, polymer-engineered AgNP-impregnated membranes are currently being explored due to the effective removal of bacteria. Mohammad and co-workers synthesized a nanohybrid polysulfone membrane with silver-decorated graphene nanoplates and this nanohybrid membrane shows excellent antibacterial properties.15 The Mauter and Zodrow groups demonstrated that synthesized nano-Ag can be surface grafted on polymeric membranes to inhibit bacterial attachment and biofilm formation on the membrane surface, as well as inactivate viruses.16,17 Cao et al., investigated antibacterial effect of AgNPs in a polyethersulfone membrane against S. aureus, S. albus and E. coli.18 Diagne et al., modified a commercially available polyethersulfone membrane by a polyelectrolyte multilayer modification method using poly(styrenesulfone), poly(diallyldimethylammonium chloride) and AgNPs, which show resistance to biofouling and inhibit bacterial growth.19 Smith et al. have shown that AgNPs embedded in porous ceramic media improve the removal and disinfection of E. coli.20 Morones et al., studied the activity of nanoscale silver particles embedded in a carbon matrix towards four types of Gram negative bacteria and they found that all types of Gram negative bacteria are inactivated due to interaction with the AgNPs.21 Taurozzi et al. found that when polysulfone (PSf) membranes are formed with AgNPs included in the casting solution by ex situ and in situ reduction methods, water permeability was increased.22 Polymer engineered AgNPs have attracted interest due to their antibacterial properties. Jang reported the synthesis of poly[2-(tert-butylaminoethyl)methacrylate] (PTBAM) embedded AgNPs by radical-mediated dispersion polymerization. The fabricated Ag/PTBAM nanofibers show enhanced antibacterial activities against both Gram negative E. coli and Gram positive S. aureus.23 An et al., studied the capability of an electrospun chitosan–polyethylene oxide membrane impregnated by silver for removal of E. coli.24 Poly(methyl methacrylate) (PMMA) has been extensively used in medical and dental applications due to its biocompatible nature. Alt et al., described the in vitro antibacterial activity of nanosilver against multi resistant bacteria and the in vitro cytotoxicity of AgNPs loaded into PMMA bone cement.25
Here, our study focuses on the in situ synthesis of AgNPs in a polymer matrix through photo-assisted processes. Taking advantage of the beneficial properties of UV rays and the antibacterial effects of Ag, we have synthesized an Ag–PMMA nanocomposite for water treatment. In the present study, PMMA was used as polymer substrate to encapsulate AgNPs. Photochemical fabrication is one of the most powerful, simple, cost-effective and convenient techniques for the synthesis of metal nanoparticles. In principle, the photochemical approach is the generation of M0 in such conditions that their precipitation is thwarted. M0 can be formed through direct photoreduction of a silver source, silver salt or complex, or reduction of silver ions using photochemically generated intermediates, such as radicals. In this one-step approach, we report a strategy involving the photoinduced formation of homogeneous AgNPs in a polymer (PMMA) stemming from a cross-linking photo-polymerization of a methyl methacrylate monomer. The effect of MMA monomer volume on the synthesis of Ag–PMMA nanocomposite has been investigated. In addition, the antibacterial properties of the Ag–PMMA nanocomposite have been evaluated against E. coli, P. aeruginosa (Gram negative) and S. aureus (Gram positive) bacteria using antibacterial activity, growth kinetics and minimum inhibitory concentration (MIC). The synthesized Ag–PMMA nanocomposite exhibited excellent antimicrobial activity toward Gram negative and Gram positive bacteria. We have made an attempt to explore the possibility of the fabricated Ag–PMMA nanocomposite as antibacterial agent for water treatment. Sludge water was collected from the Mula River (Pune, India) which contains Gram positive as well as Gram negative bacteria. To test the bactericidal action, a colloidal solution of the nanocomposite was loaded onto a membrane (the ‘treated membrane’). Then sludge water was passed through the treated membrane and effluent water was analyzed for viable bacteria. Deactivation of bacteria in the sludge water occurred by percolation followed by death confirmed by antibacterial activity and a microbial slide test. The membrane was characterized in terms of surface morphology by field emission scanning electron microscope (FE-SEM) studies.
Experimental
Materials
All the chemicals used were of analytical grade. Silver nitrate (AgNO3) (Fluka, Switzerland), methyl methacrylate (MMA) (Merck, India), dioctyl sodium sulfosuccinate (AOT), 2,2-azobis(isobutyronitrile) (AIBN) and propan-2-ol were obtained from Merck, India. All the reagents were of analytical grade and used as received, without further purification. Milli-Q deionized water was used for synthesis. To test bacterial growth, E. coli (ATCC 11775), P. aeruginosa (ATCC 47085) and S. aureus (ATCC 12600) were purchased. Millipore PVDF membranes of 13 mm diameter with 0.22 μm pore size were purchased from Merck (India). The syringe with membrane holder was used for sludge water filtration.Synthesis of the Ag–PMMA nanocomposite
In a typical procedure, a total volume of 100 mL was made by adding a silver precursor of 88.6 mL (AgNO3, 10−3 M), 2% AOT (10 mL), AIBN (0.047 g), isopropanol 1.2 mL (0.2 M), and MMA of (0.02 M) 0.2 mL to Milli-Q water. Low pressure Hg lamps of 200 W (Srinivasan–Griffin Rayonet, JSGW type) were used as a UV light source and the solution was irradiated for 24 h. This instrument comprised eight ultraviolet tubes of wavelength 253.7 Å fitted in a heavy metal enclosure with an in-built magnetic stirrer. The incident photon number of 253.7 nm light (determined by a tris(oxalato)ferrate(III) actinometer) was 5.0 × 1015 cm2 s−1. When the reaction solution was irradiated with UV light, the room temperature was controlled by using water circulation. After UV irradiation, the solution color changed from colorless to yellow, indicating the formation of PMMA embedded nanoparticles.Organic reagent test
A 0.1 M stock solution of rhodanine was prepared in water. 10 μL of rhodanine (0.1 M) solution was added to 5 mL AgNO3 (0.1 M) solution. After a regular time interval (5 min, 7 h and 24 h), UV-visible spectral study of these solutions was performed. The same experimental procedure was performed for synthesized Ag–PMMA nanocomposite.Effect of MMA monomer volume on the synthesis of nanocomposite
A total volume of 100 mL was made by adding a silver precursor of 88.6 mL (AgNO3, 10−3 M), 2% AOT (10 mL), AIBN (0.047 g), isopropanol 1.2 mL (0.2 M), and MMA of (0.02 M) 0.2/0.4/0.6/0.8/1 mL to Milli-Q water. Low-pressure Hg lamps of 200 W were used as a UV light source and the solution was irradiated for 24 h. After UV irradiation, the solution color changed from colorless to yellow, indicating the formation of PMMA embedded nanoparticles.Borohydride reduced silver nanoparticles
Silver nanoparticles (AgNPs) were prepared by reduction of 10 mL of 1 mM silver nitrate (AgNO3) with 30 mL of 2 mM ice-cold sodium borohydride solution (NaBH4). Briefly, the solution of silver nitrate was vigorously stirred with the sodium borohydride solution for an hour at room temperature. Finally, the solution color changed from colorless to yellow, indicating the formation of AgNPs.Ag–PMMA nanocomposite loaded membrane
The membrane was moistened by dipping it in a 50% ethanol–water mixture. The membrane was then placed into the membrane holder. The membrane holder was then attached to the syringe and 10 mL of Ag–PMMA nanocomposite solution was passed through the membrane (treated membrane). The membrane traps the Ag–PMMA nanocomposite on the upper side of membrane. The Ag–PMMA nanocomposite loaded membrane was then removed from the holder and dried.Analysis for silver in effluent
The effluent after passing through the treated membrane was analyzed for silver content by MP-AES. 9 mL of nitric acid (10%) was added to 1 mL effluent. This solution was kept overnight. The samples were then analyzed for total silver content by MP-AES. The sample measurements were done in triplicate. The values reported were based on a calibration curve using an Ag MP-AES standard. The emission Ag line is at 328.06 nm. The silver loss from the treated membrane in 10 mL of effluent was determined from the MP-AES values for silver concentration in the effluent water. This value was expressed as a percentage of the total silver mass contained in the treated membrane, as determined by MP-AES measurements.To quantify the amount of silver in the treated membrane, analysis was performed by acid digestion of the membrane and determination of the amount of dissolved silver using MP-AES. Briefly, approximately 100 mg of dried membrane was reacted with 5 mL of 70% nitric acid in 5 mL of water and then boiled until it disintegrated. After cooling, the suspension was filtered through a glass filter. The silver content of the effluent was measured with MP-AES.
Instrumentation
The optical absorption spectra of colloidal solutions were recorded on a UV-vis 1800 (Shimadzu) spectrophotometer. FTIR spectra were recorded on a Bruker Tensor 27 instrument. High resolution transmission electron microscopic (HRTEM) images were taken using JEOL-JEM 2100 HRTEM operated at 200 kV, for which the samples were prepared by dropping a 2 μL nanocomposite solution onto a 400-mesh icon carbon-coated copper grid and wicking off the excess sample with filter paper after 30 s and drying at room temperature. Crystallinity and crystal phases of the synthesized nanocomposite were determined by a Philips PW 1840 powder X-ray diffractometer (XRD) with Cu Kα radiation (l = 1.54178 Å) and a Bragg angle ranging from 20 to 80°. The particle size was calculated from the full width at half maximum (FWHM) of the diffracted lines using the Scherrer formula. The thermal decomposition pattern of the sample was studied by thermogravimetric analysis (TGA) using a TGA 2050 analyzer. TGA was conducted from room temperature to 900 °C in a nitrogen atmosphere. The heating rate was maintained at 10 °C min−1. Dynamic light scattering (DLS) was performed to determine hydrodynamic size and zeta potential of the Ag–PMMA nanocomposite using Malvern ZetasizerNano ZS instrument. Briefly, the samples were loaded into a pre-rinsed folded capillary cell and a voltage of 150 and 100 V was applied. The zeta potential measurements were made in triplicate. The presence of Ag–PMMA nanocomposite in the membrane was established by measuring the reflectance spectra of the treated membrane on a UV-visible diffuse reflectance spectrophotometer (DRS, JASCO V-670). Microwave plasma-atomic emission spectrometry (MP-AES, Agilent 4100) was used for the quantification of silver content. Optical microscopy images were taken on a Leica DM 2500 microscope. The microstructure of the sample was observed by FE-SEM (Nova NanoSEM 450). The samples were drop-cast on a silicon wafer and coated by chromium/gold sputtering for conductivity.Antibacterial test
The Ag–PMMA nanocomposite was tested against E. coli, P. aeruginosa and S. aureus by the well diffusion method. A bacterial suspension was cultured in nutrient broth medium at 37 °C for 24 h. Drops of bacterial suspension (100 μL) were added onto the surface of the agar and spread using a sterile glass spreader. A 7 mm diameter well was made on nutrient agar plate with the help of a gel puncture. 25 μL of Ag–PMMA nanocomposite and PMMA solution (without Ag) were added to the well, and then the plates were placed in an incubator at 37 °C for 24 h. The same procedure was followed to test the antibacterial activity of the treated membrane. The standard error was calculated using three replicate experiments.The minimum inhibitory concentration (MIC) test was performed as follows. Sterilized LB agar solutions (5 mL) were inoculated with bacterium (105–106 CFU). The synthesized nanocomposite was then added to the bacterial suspensions at different concentrations (5, 10, 15, 20 and 25 μL) and incubated at 37 °C with shaking at 150 rpm for 24 h. Bacterial growth was measured as an increase in absorbance at 600 nm. The experiments also included a positive control (a flask containing the nanocomposite and nutrient media) and a negative control (flask containing inocula and nutrient media). Three replicates of this experiment were performed.
For the kinetic tests, E. coli, P. aeruginosa and S. aureus suspensions were prepared. The culture medium of each bacterium was inoculated with bacteria and incubated overnight at 37 °C. Bacterial growth rates were measured by monitoring the optical density at 600 nm (OD600) using a spectrophotometer. The incubated bacteria were inoculated in the fresh media and grown at 37 °C with 200 rpm of shaking to an OD600 of 0.1. Various concentrations of Ag–PMMA nanocomposites (from 1 to 25 μL) were then added to the culture, and the OD600 was measured over time. This experiment was repeated three times.
Ag–PMMA nanocomposite interaction with E. coli bacteria
To demonstrate the mechanism of the biocidal action of the Ag–PMMA nanocomposite, E. coli bacterial cells (each at 106–107 CFU mL−1) were treated with the Ag–PMMA nanocomposite (25 μL) for 6 h. Untreated E. coli cells were used as control. The treated cells were harvested by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min) and washed thrice with Milli-Q water to remove the loosely bound Ag–PMMA nanocomposite on the bacterial surface. A series of pre-treatment steps were followed for FE-SEM analysis. Primary fixation of bacterial cells was achieved using 2.5% glutaraldehyde for 1 h. Then, samples were subsequently dehydrated with a graded ethanol series (30%, 50%, 70%, 90%, 95% and 100%).
000 rpm, 10 min) and washed thrice with Milli-Q water to remove the loosely bound Ag–PMMA nanocomposite on the bacterial surface. A series of pre-treatment steps were followed for FE-SEM analysis. Primary fixation of bacterial cells was achieved using 2.5% glutaraldehyde for 1 h. Then, samples were subsequently dehydrated with a graded ethanol series (30%, 50%, 70%, 90%, 95% and 100%).
Results and discussion
The synthesis of PMMA-embedded AgNPs was accomplished by UV irradiation of the solution of silver nitrate, AOT and MMA. As a result of UV-irradiation, simultaneous polymerization and reduction of metallic silver took place. Under UV irradiation, polymerization of MMA to PMMA using AIBN (reductant radical inhibitor) occurred. Also, AIBN undergoes partial electron transfer with silver salt, thus reduction of metallic silver occurred in the presence of UV irradiation. The reduced silver atoms were stabilized by the AOT surfactant coordinating the sulfonic group of AOT.26 The stabilized AgNPs have a strong interaction with oxygen in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the PMMA polymer. The high interaction of AgNPs with carbonyl oxygen (C
O groups of the PMMA polymer. The high interaction of AgNPs with carbonyl oxygen (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) compared to that of the C–O in the ester functional group is due to the higher negativity of the charge density for the C
O) compared to that of the C–O in the ester functional group is due to the higher negativity of the charge density for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O than C–O in the polymer matrix.27,28
O than C–O in the polymer matrix.27,28
To clarify the morphology of polymer embedded AgNPs, TEM analysis was carried out. Fig. 1 shows a TEM image of the synthesized nanocomposite. TEM analysis confirmed that AgNPs of average 17.30 ± 0.4 nm in diameter were finely embedded throughout the polymer nanorods. The Ag–PMMA nanocomposite shows an average diameter of 0.4 μm and a length of 2.2 μm with a standard deviation of ±2.4 nm (Fig. S1, ESI†). Additionally, the synthesized nanocomposite possesses a smooth surface morphology (Fig. 1a–g), which indicates that the AgNPs were not located on the surface but instead were embedded inside the PMMA nanorods. To gain further insight into the morphology, HRTEM was performed on polymer embedded AgNPs (Fig. 1h). The synthesized nanocomposite exhibited internal highly ordered lattice fringes with a lattice spacing of 0.23 nm, corresponding to the (111) lattice plane of Ag. The interplanar distance of the nanosilver (111) plane is in good agreement with the (111) d-spacing of bulk Ag (0.2359 nm).29 The favored alignment of AgNPs in PMMA is at the (111) plane. This can be elucidated from the perspective of thermodynamics, since the preferred orientations of solid particles are known to be perpendicular in direction to the plane of lowest surface energy, which corresponds to the most densely packed planes for metallic materials.30,31 The SAED pattern confirms the polycrystalline nature of the AgNPs (Fig. 1i).
 | ||
| Fig. 1 TEM micrographs of (a and g) PMMA embedded AgNPs with bright field (a–d, f and g) and dark field (e) modes. (h) HR-TEM image and (i) SAED pattern of polymer embedded AgNPs. | ||
The crystalline character of the Ag–PMMA nanocomposite was supported by XRD analysis (Fig. S2, ESI†). The synthesized nanocomposite exhibits four distinct diffraction peaks at 2θ values of 38.1°, 44.3°, 64.4°, and 77.3° correspond to the (111), (200), (220), and (311) planes of the Ag crystal, respectively (JCPDS card number 4-0783). All the diffraction peaks can be indexed to the planes of face-centered-cubic (fcc) silver, with a lattice constant of about 4.08 Å.32 The XRD results demonstrated that the preferred growth plane of the particles is the (111) lattice plane. The crystallite size (34.4 nm, average particle size) of the synthesized nanocomposite was calculated from the FWHM of the peaks of silver by the Scherrer equation.
The size distribution of the Ag–PMMA nanocomposite was measured by DLS. The average particle size obtained from DLS data is 178 nm (Fig. S3, ESI†). DLS analysis indicates that the effective hydrodynamic diameter of the Ag–PMMA nanocomposite measured by DLS is larger than the hard core sizes measured by TEM. DLS analysis includes the ligand shell and determines the hydrodynamic size, while TEM analysis provides the information of the metallic core of particle.33 Dynamic Light scattering (DLS) is a technique for measuring the size of particles typically in the sub micron region. DLS measures Brownian motion and relates this to the size of the particles. Brownian motion is the random movement of particles due to the bombardment by the solvent molecules that surround them. Normally DLS is concerned with measurement of particles suspended within a liquid. The larger the particle, the slower the Brownian motion will be. Smaller particles are moved further by the solvent molecules and move more rapidly. The velocity of the Brownian motion is defined by a property known as the translational diffusion coefficient (D). The size of a particle is calculated from the translational diffusion coefficient by using the Stokes–Einstein eqn (1);
 | (1) |
The diameter measured in DLS signifies how a particle diffuses within a fluid so it is referred to as the hydrodynamic diameter. It is the diameter of a sphere that has the same translational diffusion coefficient as the particle. The translational diffusion coefficient mainly depends on the size of the particle core, the surface structure, the concentration, the type of ions in the medium and the ionic strength of the medium as well. Any change to the surface of a particle that affects the diffusion speed will correspondingly change the apparent size of the particle. An adsorbed polymer layer projecting out into the medium may reduce the diffusion speed. The nature of the surface and the polymer, as well as the ionic concentration of the medium can affect the polymer conformation, which in turn can change the apparent size by several nanometres.33
DLS measures the hydrodynamic diameter of the whole solution components viz. in situ AgNPs, including the Ag core, capping agents and any other molecules sorbed to the surface, and layers of solvent molecules that are associated with the NPs during Brownian motion. These components are assumed to conform to a spherical geometry. During DLS measurements, Rayleigh scattering dictates that the light intensity scattered by a NP is proportional to its diameter raised to the sixth power, and thus larger diameter particles will dominate the intensity signal.33
Using DLS we get the hydrodynamic radius of the particle, whereas by TEM we get an estimation of the projected area diameter. According to DLS theory, when a dispersed particle moves through a liquid medium, a thin electric dipole layer of the solvent adheres to its surface. This layer influences the movement of the particle in the medium. Thus, the hydrodynamic diameter gives us information of the inorganic core along with any coating material and the solvent layer attached to the particle as it moves under the influence of Brownian motion. While estimating the size by TEM, due to the difference in the sample preparation method, the hydration layer is absent; hence, we get information only about the inorganic core. The projected area diameter estimated by TEM is theoretically defined as the area of a sphere having the same area as the projected area of the particle resting in a stable position. Sometimes, due to poor TEM contrast, the size measurement of the coating layer (if present) could be underestimated or missed. Hence, the hydrodynamic diameter is always greater than the size estimated by TEM.
The Ag–PMMA nanocomposite was further examined by TGA in order to obtain quantitative information on the silver content (Fig. S4, ESI†). It was observed from the TGA curve that the two dominant weight losses of the sample occurred in the temperature region between 158–506 °C (Fig. S4, ESI†). There was almost no weight loss after 506 °C. The total weight loss was 90.77%, resulting in a silver content of 9.33%. Marning and coworkers also reported that the unsaturated ends (PMMA) are responsible for two step weight losses around 180 °C and 270 °C for silver–polymer nanoparticles. They observed two step weight losses mainly in the temperature range from 158 to 506 °C with silver–polymer nanoparticles.29,34 Earlier study has reported that the thermal stability of PMMA is improved due to the presence of silver. The presence of a small amount of Ag in the polymer matrix confines the motion of the polymer chains, which renders improved thermal stability.35
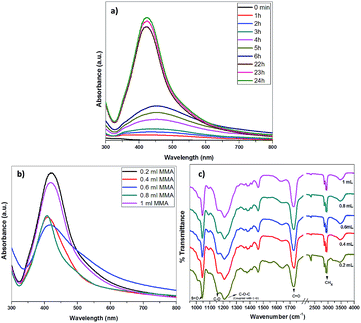
The characteristic surface plasmon resonance (SPR) spectra of metal nanoparticles provides a convenient tool to monitor their formation and gain insight into the particle size and shape. The optical property of the Ag–PMMA nanocomposite was analyzed by UV-visible spectroscopy. Fig. 2a depicts the absorption band at 421 nm, which is a characteristic SPR band for AgNPs. It is known that SPR absorption of nanoparticles is sensitive to geometric parameters, aggregation and the surrounding matrix. The SPR band starts to appear after 1 h of irradiation and grows with time. The formation of Ag–PMMA nanocomposite was completed within 24 h (Fig. 2a); further irradiation up to 48 h showed no change in the SPR band. This result indicates that 24 h is the optimum irradiation time for the synthesis of the Ag–PMMA nanocomposite.
In order to verify the role of AOT in the synthesis, a blank system containing only silver ion and MMA (without AOT) was examined. There was no formation of thr Ag–PMMA nanocomposite and no color change of solution. From this experiment, it can be concluded that AOT acts as a stabilizing agent to prohibit aggregation of silver. Also, to ensure the compatibility of the solution, it was ensured that AOT and MMA with AIBN do not interact before irradiation. More information about the role of the AOT surfactant is provided in the ESI section (Fig. S5, ESI†).
The effect of MMA monomer volume on the formation Ag–PMMA nanocomposite was studied by UV-visible and FTIR spectroscopy (Fig. 2). UV-visible study demonstrates that, as the volume of MMA monomer increases (0.2 to 1 mL), the SPR band of the Ag–PMMA nanocomposite becomes broader (Fig. 2b). This result indicates that the 0.2 mL volume of MMA is the optimum volume for the synthesis of Ag–PMMA nanocomposite. The FTIR spectra of Ag–PMMA nanocomposite presented in Fig. 2c, shows a broad band around 3399 cm−1, indicating the presence of an –OH group. Prominent bands at 2955, 2928 and 2867 cm−1 are attributed to C–HX bending and stretching vibration.36–39 The strong bands at 1728 cm−1 and 1650 cm−1 are ascribed to the carbonyl stretching vibration and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration, respectively.36,37 The bands at 1460 cm−1 and 1385 cm−1 are associated with CH3 and C–H bending modes. The band at 1211 cm−1 corresponds to C–C–O coupled with C–O stretching,36,37 whereas bands at 1160 cm−1 and 749 cm−1 are accredited to the C–O symmetrical stretching modes of ester groups from PMMA and the rocking vibration of CH2, respectively.38 The sharp band at 1044 cm−1 is assigned to the S
C stretching vibration, respectively.36,37 The bands at 1460 cm−1 and 1385 cm−1 are associated with CH3 and C–H bending modes. The band at 1211 cm−1 corresponds to C–C–O coupled with C–O stretching,36,37 whereas bands at 1160 cm−1 and 749 cm−1 are accredited to the C–O symmetrical stretching modes of ester groups from PMMA and the rocking vibration of CH2, respectively.38 The sharp band at 1044 cm−1 is assigned to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the sulfonate group present in the AOT molecules.26 However, the strong bands at 1728 cm−1 correspond to the carbonyl stretching vibration, which becomes sharper with the increase in the volume of the MMA monomer. FTIR study demonstrated that binding takes place through carbonyl groups from PMMA with AgNPs.
O stretching vibration of the sulfonate group present in the AOT molecules.26 However, the strong bands at 1728 cm−1 correspond to the carbonyl stretching vibration, which becomes sharper with the increase in the volume of the MMA monomer. FTIR study demonstrated that binding takes place through carbonyl groups from PMMA with AgNPs.
To check the stability, an aggregation test for borohydride reduced silver nanoparticles (AgNPs–B) and Ag–PMMA nanocomposites in presence of light and absence of light (dark) was carried out for 5 days (Fig. 3). Under white-light (60 W) conditions, it was observed that the color of AgNPs–B turned from yellow to green after 5 days. The corresponding UV-visible spectra of AgNPs–B show SPR bands at 401 nm (intensity decreased) and 650 nm, suggesting that the AgNPs were aggregated (Fig. 3a).40,41 This result indicates that the aggregation process was light sensitive. Meanwhile, the AgNPs–B stored in the dark exhibited no color change, as indicated by no shift in the SPR band position. Under the same conditions, aggregation tests for the Ag–PMMA nanocomposite was carried out. The UV-visible spectra of the Ag–PMMA nanocomposite were nearly unchanged when stored either in the dark or in the light for 5 days (Fig. 3b). Based on these data, it can be concluded that the Ag–PMMA nanocomposite has a lower tendency toward aggregation than AgNPs–B because the polymer substrate prevented aggregation of the embedded AgNPs.42 The stability was also checked using the zeta potential. A negative/positive zeta potential value indicates the degree of repulsion between charged particles in a nanosuspension. A more negative zeta potential value indicates a stronger electrostatic repulsion force between nanoparticles and better stability. The Ag–PMMA nanocomposite shows a zeta potential of −63.9 mV confirming the high stability of the nanocomposite (Fig. S6, ESI†).
An organic reagent test was performed to examine the oxidation of the Ag–PMMA nanocomposite. It has been reported that metallic silver can be oxidized to silver ion.43 Particularly, for the detection of silver salts, rhodanine is used because it is a selective and sensitive reagent towards silver ion. After addition of an aqueous solution of rhodanine into a silver nitrate (AgNO3) solution, an immediate color change occurred (colorless to light yellow) which gradually changed further into a brown–black precipitate due to the formation of an Ag–rhodanine complex (Fig. 4). Stephen and co-workers reported that precipitation occurred due to the involvement of the acidic imino-hydrogen group of rhodanine with a silver ion.44 The same rhodanine solution, however, when added to an Ag–PMMA nanocomposite solution showed no color change; only the color became faint after 24 h without any precipitation. This result indicates that a lesser amount of AgNPs in polymer matrix was oxidized (Ag+) since most of the particles exist in the Ag0 state.
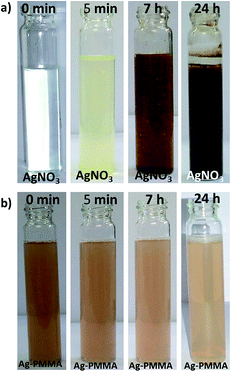 | ||
| Fig. 4 Photographic images of (a) silver nitrate and (b) Ag–PMMA nanocomposite solution as a function of rhodanine addition with time. | ||
Antibacterial activity of Ag–PMMA nanocomposite
The antibacterial activity of the Ag–PMMA nanocomposite were investigated against E. coli, P. aeruginosa and S. aureus using an agar-gel method. The antibacterial properties were measured by evaluating the zone of inhibition (ZoI) around the disk after incubation at 37 °C (Fig. S7, ESI†). The diameter of the zone of inhibition for the Ag–PMMA nanocomposite (25 μL) was 14 ± 0.25, 13 ± 0.48 and 10 ± 0.36 mm against E. coli, P. aeruginosa and S. aureus, respectively (Fig. S7b, ESI†). However, the PMMA system (without Ag) showed no zone of inhibition against E. coli, P. aeruginosa and S. aureus (Fig. S7a, ESI†). These results indicated that the Ag–PMMA nanocomposite exhibited antibacterial properties. Jang and coworkers have found similar results, that the nanometer-sized PTBAM polymer nanofibers provided a large surface area for more effective antimicrobial performance.23To study the growth kinetics of E. coli, P. aeruginosa and S. aureus bacteria with the Ag–PMMA nanocomposite a bacterial inhibition growth curve was used. The dynamics of bacterial growth curve were monitored in liquid LB broth. Time-dependent changes in the bacterial growth were monitored at a regular interval of 2 h (up to 12 h) by measuring the OD (at 600 nm) of the control (without Ag–PMMA nanocomposite), and bacterial solutions supplemented with Ag–PMMA nanocomposite (0.5 μg mL−1) are shown in Fig. S8, ESI.† Bacterial cell growth enhances the turbidity of the liquid medium and as a result, the absorption increases. Ag–PMMA nanocomposite caused a growth delay of the bacterial cells and the slope of the bacterial growth curve continuously decreased with time (Fig. S8, ESI†). An MIC test was also performed to quantify the antibacterial activity of the Ag–PMMA nanocomposite against E. coli, P. aeruginosa and S. aureus bacteria (Fig. S9, ESI†). The MIC value for the Ag–PMMA nanocomposite was found to be in the range of 0.032 to 0.125 μg mL−1 for E. coli, P. aeruginosa and S. aureus bacteria. These results demonstrate that the Ag–PMMA nanocomposite inhibits bacterial growth.45
Antibacterial activity (MIC and growth curve, ZoI) results show that Ag–PMMA nanocomposite was more lethal to E. coli and P. aeruginosa than to S. aureus bacteria. This is because Gram positive bacteria have a large number of free amines and carboxyl groups on their surfaces, while Gram negative have the capability to protect themselves from antimicrobial agents. Also, the different strength of the antibacterial activities of Ag–PMMA nanocomposite towards Gram positive (E. coli and P. aeruginosa) and Gram negative (S. aureus) bacteria arises due to differences in the cell structure of the bacteria. Gram positive bacteria possess a cell wall but Gram negative bacteria do not. Also both bacteria have different physiologies, activities and metabolic rates.46
Ag–PMMA nanocomposite interaction with E. coli bacteria
The precise mechanism of action of silver on the microbes is still not recognized. The literature reports that AgNPs are first attached to the cell membrane and penetrate inside the bacteria.47 In general silver has a greater propensity to react with sulfur- or phosphorus-containing soft bases (R–S–R, R–SH, RS– or PR3). Thus, sulfur-containing proteins in the cell membrane and inside the cells’ phosphorus-containing elements like DNA are potential binding sites for AgNPs. The AgNPs preferably attack the respiration chain; cell division finally leading to cell death.48In order to investigate the interaction behavior of the Ag–PMMA nanocomposite with E. coli bacteria, FE-SEM imaging was conducted (Fig. 5). E. coli bacterial cells treated (Fig. 5b) with the Ag–PMMA nanocomposite show antagonistic effects as compared to the untreated cells (control) (Fig. 5a). Based on FE-SEM analysis, possible mechanisms of bactericidal action may be hypothesized. As shown in the micrographs (Fig. 5b–e), Ag–PMMA nanocomposite treated cells appeared to show a typical shape and a few of the cells were severely damaged (indicated by the arrow in Fig. 5b). Membrane disruption may increase the cell permeability and permit intracellular material to come out, eventually causing cell death (encircled in Fig. 5c and e). Sondi reported that AgNPs have the ability to anchor to the bacterial cell wall and subsequently penetrate it, thereby causing structural changes in the cell membrane, such as permeability of the cell membrane and death of the cell.49 The results clearly indicate that the Ag–PMMA nanocomposite is a promising candidate for antibacterial applications due to its dual mode of antibacterial action: contact-killing and release of metal ions.
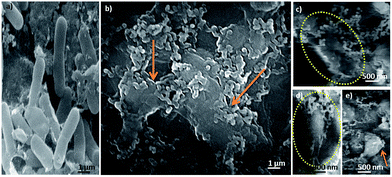 | ||
| Fig. 5 FE-SEM micrographs of E. coli bacterial cells (a) untreated (control) and (b–e) treated with the Ag–PMMA nanocomposite. Micrographs of (b–e) damaged E. coli cells with ruptured morphology. | ||
Ag–PMMA nanocomposite loaded membrane
The treated membrane was prepared by passing the Ag–PMMA nanocomposite solution through the membrane using a syringe. The membrane changed color from white to yellow after the Ag–PMMA nanocomposite solution was passed through it (inset Fig. 6b). Characterization of treated membrane was carried out using DRS, FE-SEM and FTIR. The DRS spectrum of the treated membrane (Fig. S10, ESI†) shows a peak at 360 nm, which corresponds to silver. An optical image of a blank membrane (untreated membrane) shows a porous nature before treatment with the nanocomposite (Fig. 6a), while the membrane after treatment with the Ag–PMMA nanocomposite retains its porosity, only the color of the membrane changes from white to yellow (Fig. 6b). The morphology of the untreated and treated membrane was determined by FE-SEM (Fig. 6c and d). The FE-SEM micrograph of the untreated membrane shows porous morphology (Fig. 6c), however the surface of the treated membrane was covered with a rod shaped nanocomposite. This nanocomposite showed an average diameter of 82 nm and a length of 317.4 nm with a standard deviation of ±3.7 nm. More FE-SEM images of treated membrane are shown in Fig. S11, ESI.† FITR study of treated membrane demonstrated that, after loading of the Ag–PMMA nanocomposite no shift in frequency was observed; only the intensity of bands (2978 and 1719 cm−1) changed, which suggests physical adsorption (Fig. S12, ESI†).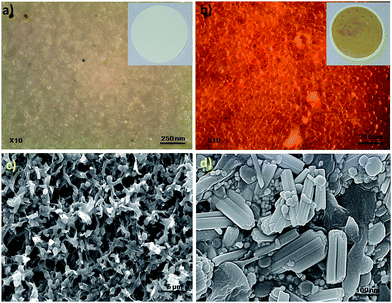 | ||
| Fig. 6 Optical and FE–SEM images of the untreated (a and c) and treated membrane (b and d). The inset shows photographic images of the untreated and treated membranes. | ||
Additionally, the antibacterial activity of the treated membrane was investigated against E. coli and S. aureus using an agar-gel method. The antibacterial properties were measured by evaluating the zone of inhibition around the disk after incubation at 37 °C. The treated membrane (1 cm) shows antibacterial activity with a zone of inhibition of 13 ± 0.33 and 12 ± 0.57 mm for E. coli and S. aureus (Fig. S13, ESI†). This result indicated that the treated membrane possesses antibacterial properties. However, the untreated membrane does not show any antibacterial properties (data not shown here).
Bactericidal effectiveness of the treated membrane for water treatment
The treated membrane delivered rapid and effective bactericidal activity as the model microorganisms present in sludge water were poured through the treated membrane. The average percolation time for 100 mL of sludge water was 10 min. Sludge water contains Gram positive and Gram negative bacteria, which was confirmed by microscopy (Fig. S14, ESI†). When sludge water was passed through the untreated membrane, there was little reduction in bacterial growth (Fig. 7b) compared to the original sludge water (Fig. 7a). Interestingly, the removal of lesser percentage of microorganisms was achieved by the untreated membrane, which indicates that the membrane without nanocomposite can separate microorganisms to a certain extent by size exclusion. This result demonstrated that the majority of microorganisms have a larger size than the pore size of the membrane. Also, diluted (10× to 100×) sludge water was passed through the untreated membrane and bacterial growth was evaluated on an agar plate (Fig. S15, ESI†). Sludge water (50×) was poured through the treated and untreated membrane incubated for 24, 48 and 96 h to monitor bacterial growth. However it was observed that the untreated membrane shows an increase in bacterial growth with time (Fig. 7d–f), whereas the treated membrane shows a reduction in bacterial growth at 24 h and 48 h incubation (Fig. 7g and h). Complete deactivation of bacterial growth occurred at 96 h incubation (Fig. 7i). Fig. 7c depicts the absence of Gram positive and Gram negative bacteria in the effluent by microbial slide test (72–108 h). These results indicated that complete deactivation of bacteria by the treated membrane occurred. Consequently, the filter effluent contains dead bacteria, which indicates that the treated membrane exhibits antibacterial activity.The silver content of the effluent water was analyzed because of possible human health effects from silver exposure to the environment and ecosystem.50 The silver content in the effluent water was 0.09 ppm measured by MP-AES. The quantitative estimation of silver content in the effluent of the diluted sludge water (10× to 100×) of the treated membrane is summarized in Table S1, ESI.† The acid digestion of the treated membrane (0.1 g) showed a silver content of 1.19 ppm. Meanwhile, the untreated membrane (without passing bacteria through the Ag–PMMA loaded membrane) shows a silver content of 1.28 ppm. However, MP-AES analysis indicated that the untreated membrane (blank membrane) contains no silver. MP-AES analysis indicates a lesser amount of leaching of silver (0.1 ppm) from the treated membrane, which suggests that Ag–PMMA nanocomposite loaded membrane is suitable for water treatment.
Conclusions
AgNPs were embedded in a PMMA matrix by in situ photo assisted polymerization of MMA using AIBN to initiate the polymerization process. The fabricated nanocomposite exhibited a lower tendency toward aggregation compared to borohydride synthesized AgNPs. A systematic evaluation of the antibacterial activity of the composite nanoparticles was carried out and showed excellent bactericidal properties against Gram positive and Gram negative bacteria. Importantly, deactivation of bacteria by percolation through the fabricated nanocomposite loaded membrane occurred. Therefore, the filter effluent contains dead bacteria, which indicates that treated membrane exhibits antibacterial activity. MP-AES analysis estimated that the amount of silver leaching from the treated membrane is less than 0.1 ppm. The bactericidal action of the Ag–PMMA nanocomposite loaded membrane remains, however, to be tested on large scale. Hopefully, this work will provide an effective, efficient, and economic method for water treatment.Abbreviations
| Ag–PMMA | Silver–poly(methyl methacrylate) nanocomposite |
| FTIR | Fourier transform infrared spectroscopy |
| HRTEM | High-resolution transmission electron microscopy |
| XRD | X-Ray diffractometer |
| FWHM | Full width at half maximum |
| TGA | Thermogravimetric analysis |
| DLS | Dynamic light scattering |
| DRS | Diffuse reflectance spectrophotometry |
| FE-SEM | Field emission scanning electron microscope |
| MP-AES | Microwave plasma-atomic emission spectrometry |
| MIC | Minimum inhibitory concentration |
| E. coli | Escherichia coli |
| P. aeruginosa | Pseudomonas aeruginosa |
| S. aureus | Staphylococcus aureus |
| Treated membrane | Ag–PMMA nanocomposite solution was passed through the membrane |
| Untreated membrane | Blank membrane |
Acknowledgements
Ms Shubhangi is thankful to S. P. Pune University for financial support. A. A. Khan and the authors acknowledge the Institute of Bioinformatics and Biotechnology, Savitribai Phule Pune University for providing the infrastructure for conducting biological assays. The authors are also thankful to the Venture Center, NCL, Pune for MP-AES analysis and Ms Edna Joseph for analysis as well as helpful discussion related to MP-AES. The authors are thankful to UGC-UPE Phase II (Bio-Nano) for financial support. The authors are also thankful to the Centre Instrumentation Facility (CIF), SPPU, Pune for FE-SEM analysis.References
- Global Risks 2015-Reports-World Economic Forum, reports. http://weforum.org/global-risks-2015/press-releases.
- W. Lee and P. Westerhoff, Water Res., 2009, 43, 2233–2239 CrossRef CAS PubMed.
- H. W. Glaze, Environ. Sci. Technol., 1987, 21, 224–230 CrossRef PubMed.
- S. P. Mukherjee and K. A. Ray, Chem. Eng. Technol., 1999, 22, 253–260 CrossRef.
- K. Gopal, S. S. Tripathy, J. L. Bersillon and S. P. Dubey, J. Hazard. Mater., 2007, 140, 1–6 CrossRef CAS PubMed.
- X. Qu, P. J. J. Alvarez and Q. Li, Water Res., 2013, 47, 3931–3946 CrossRef CAS PubMed.
- X. L. Qu, J. Brame, Q. Li and J. J. P. Alvarez, Acc. Chem. Res., 2013, 46, 834–843 CrossRef CAS PubMed.
- F. Meng, S. RChae, A. Drews, M. Kraume and H. S. Shin, Water Res., 2009, 43, 1489–1512 CrossRef CAS PubMed.
- M. Mukherjee and S. De, Environ. Sci.: Water Res. Technol., 2015, 1, 204–217 CAS.
- S. Agnihotri, G. Bajaj and S. Mukherji, Nanoscale, 2015, 7, 7415–7429 RSC.
- N. Musee, M. Thwala and N. Nota, J. Environ. Monit., 2011, 13, 1164–1183 RSC.
- Z. Sheng and Y. Liu, Water Res., 2011, 45, 6039–6050 CrossRef CAS PubMed.
- A. García, L. Delgado, J. A. Torà, E. Casals, E. González, V. Puntes, X. Font, J. Carrera and A. Sánchez, J. Hazard. Mater., 2012, 199–200, 64–72 CrossRef PubMed.
- P. Dallas, V. K. Sharma and R. Zboril, Adv. Colloid Interface Sci., 2011, 166, 119–135 CrossRef CAS PubMed.
- E. Mahmoudi, L. Y. Ng, M. M. Ba-Abbad and A. W. Mohammad, Chem. Eng. J., 2015, 277, 1–10 CrossRef CAS.
- M. S. Mauter, Y. Wang, K. C. Okemgbo, C. O. Osuji, E. P. Giannelis and M. Elimelech, ACS Appl. Mater. Interfaces, 2011, 3, 2861–2868 CAS.
- K. Zodrow, L. Brunet, S. Mahendra, D. Li, A. Zhang, Q. L. Li and P. J. J. Alvarez, Water Res., 2009, 43, 715–723 CrossRef CAS PubMed.
- X. Cao, M. Tang, F. Liu, Y. Nie and C. Zhao, Colloids Surf., B, 2010, 81, 555–562 CrossRef CAS PubMed.
- F. Diagne, R. Malaisamy, V. Boddie, R. Holbrook, B. Eribo and K. Jones, Environ. Sci. Technol., 2012, 46, 4025–4033 CrossRef CAS PubMed.
- D. Ren and J. Smith, Environ. Sci. Technol., 2013, 47, 3825–3832 CrossRef CAS PubMed.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramirez and M. J. Yacaman, Nanotechnology, 2005, 16, 2346–2353 CrossRef CAS PubMed.
- S. Taurozzi, H. Arul, V. Z. Bosak, A. F. Burban, T. C. Voice, M. L. Bruening and V. V. Tarabara, J. Membr. Sci., 2008, 325, 58–68 CrossRef.
- J. Song, H. Kang, C. Lee, S. H. Hwang and J. Jang, ACS Appl. Mater. Interfaces, 2012, 4, 460–465 CAS.
- J. An, H. Zhang, J. Zhang, Y. Zhao and X. Yuan, Colloid Polym. Sci., 2009, 287, 1425–1434 CAS.
- V. Alt, T. Bechert, P. Steinrücke, M. Wagener, P. Seidel, E. Dingeldein, E. Domann and R. Schnettler, Biomaterials, 2004, 25, 4383–4391 CrossRef CAS PubMed.
- S. Mandal, S. K. Arumugam, R. Pasricha and M. Sastry, Bull. Mater. Sci., 2005, 28, 503–510 CrossRef CAS.
- K. M. Abyaneh, S. Jafarkhani and S. K. Kulkarni, J. Exp. Nanosci., 2011, 6, 159–173 CrossRef.
- H. J. Kim, H. S. Joo, C. K. Kim, J. Won and S. Y. Kang, Macromol. Res., 2003, 11, 375–381 CrossRef.
- P.-Y. Silvert, R. Herrera-Urbina and K. Tekaia-Elhsissen, J. Mater. Chem., 1997, 7, 293–299 RSC.
- S. Fukuda, S. Kawamoto and Y. Gotoh, Thin Solid Films, 2003, 442, 117–120 CrossRef CAS.
- J. Herrero and C. Guillén, Vacuum, 2002, 67, 611–616 CrossRef CAS.
- Y. Sun, B. Gates, B. Mayers and Y. Xia, Nano Lett., 2002, 2, 165–168 CrossRef CAS.
- R. I. MacCuspie, K. Rogers, M. Patra, Z. Suo, J. A. Allen, N. M. Martin and A. VHackley, J. Environ. Monit., 2011, 13, 1212–1226 RSC.
- E. L. Manring, D. Y. Sogah, M. Gordon and M. G. Cohen, Macromolecules, 1989, 22, 4652–4654 CrossRef.
- S. Hsu, C.-W. Chou and S.-M. Tseng, Macromol. Mater. Eng., 2004, 289, 1096–1101 CrossRef CAS.
- D. N. Singho, A. N. Lah, R. M. Johan and R. Ahmad, Int. J. Electrochem. Sci., 2012, 7, 5596–5603 Search PubMed.
- D. N. Singho, M. R. Johan and C. A. N. Lah, Nanoscale Res. Lett., 2014, 9, 42 CrossRef PubMed.
- G. Duan, C. Zhang, A. Li, X. Yang, L. Lu and X. Wang, Nanoscale Res. Lett., 2008, 3, 118–122 CrossRef CAS PubMed.
- S. Ramesh, H. K. Leen, K. Kumutha and A. K. Arof, Spectrochim. Acta, Part A, 2007, 66, 1237–1242 CrossRef CAS PubMed.
- A. Henglein, Chem. Mater., 1998, 10, 444–450 CrossRef CAS.
- V. Dal Lago, L. França de Oliveira, K. De Almeida Gonçalves, J. Kobarg and M. Borba Cardoso, J. Mater. Chem., 2011, 21, 12267–12273 RSC.
- K. Rege, N. R. Raravikar, D.Y. Kim, L. S. Schadler, P. M. Ajayan and J. S. Dordick, Nano Lett., 2003, 3, 829–832 CrossRef CAS.
- R. Kumar and H. Münstedt, Biomaterials, 2005, 26, 2081–2088 CrossRef CAS PubMed.
- W. I. Stephen and A. Townshend, J. Chem. Soc., 1965, 3738–3746, 10.1039/JR9650003738.
- M. A. Ansari, H. M. Khan, A. A. Khan, A. Malik, A. Sultan, M. Shahid, F. Shujatullah and A. Azam, Biol. Med., 2011, 3, 141–146 CAS.
- K. A. Ojha, S. Forster, S. Kumar, S. Vats, S. Negi and I. Fischer, J. Nanobiotechnol., 2013, 11, 42–49 CrossRef PubMed.
- Q. L. Feng, J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim and J. O. Kim, J. Biomed. Mater. Res., 2000, 52, 662–668 CrossRef CAS PubMed.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef CAS PubMed.
- P. Drake and K. Hazelwood, Ann. Occup. Hyg., 2005, 49, 575–585 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: TEM micrograph, XRD, DLS, TGA, role of AOT, and zeta potential of Ag–PMMA nanocomposite; zone of inhibition, bacterial growth curve, and MIC plot of Ag–PMMA nanocomposite; DRS spectra and FE-SEM micrographs of treated membrane; FTIR spectra treated and untreated membrane; zone of inhibition of treated membrane; microscopy image of Gram positive and Gram negative bacteria; bacterial growth of untreated membrane; and MP-AES analysis of effluent. See DOI: 10.1039/c6ra08397h |
| This journal is © The Royal Society of Chemistry 2016 |