ZrO2 supported Nano-ZSM-5 nanocomposite material for the nanomolar electrochemical detection of metol and bisphenol A†
Abstract
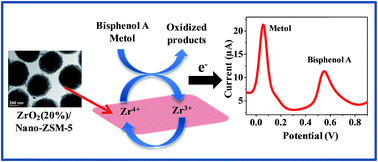
ZrO2 decorated nanocrystalline ZSM-5 nanocomposites with different weight ratios were prepared by the calcination of a physical mixture of nanocrystalline ZrO2 and Nano-ZSM-5. The material was characterized by the complementary combination of X-ray diffraction, N2-adsorption, and transmission/scanning electron microscopic techniques. An electrochemical sensor based on the ZrO2 supported nanocrystalline zeolite was developed for the nanomolar detection of organic water pollutants metol and bisphenol A with high electrocatalytic activity, stability, sensitivity, and selectivity. Under the optimum conditions, a wide linear range was obtained from 4 nM to 800 μM and 6 nM to 600 μM with a limit of detection of 1 nM and 3 nM for metol and bisphenol A, respectively. The analytical performance of the developed sensor was demonstrated for the determination of metol and bisphenol A in different real samples such as river water, metol in photographic solution, and bisphenol A in baby bottles with satisfactory results.


 Please wait while we load your content...
Please wait while we load your content...