Nanosilver rainbow: a rapid and facile method to tune different colours of nanosilver through the controlled synthesis of stable spherical silver nanoparticles†
Abstract
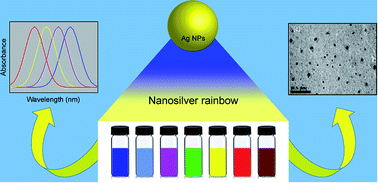
A rapid and simple one-pot reaction to synthesize stable, spherically shaped silver nanoparticles (AgNps) of different sizes producing distinct optical properties in aqueous solution at ambient temperature has been developed. Each system contains various sizes of silver nanoparticles showing rainbow colours with the peak wavelength of the absorption spectra ranging from 400 to 750 nm. Seven different colours of nano silver were developed through the controlled synthesis of spherical silver nanoparticles using silver nitrate as the metal precursor. Sodium borohydride was used as the main reducing agent and trisodium citrate and hydrazine sulphate were used as the stabilizing and auxiliary reducing agents respectively. The colour of the solution was controlled by varying the concentrations of reagents and the optimum conditions for all the colours are reported. Characterization of silver nanoparticles was carried out using UV-visible spectrophotometry and Transmission Electron Microscopy (TEM). Factors affecting the formation of different sizes of silver nanoparticles, such as silver nitrate concentration, reducing agent concentrations, reaction temperature and reaction pH are also reported. The stability of these coloured silver colloidal solutions was also investigated at different temperatures and the most stable temperature was found to be 4 °C, while the optimum pH to synthesize distinctively coloured silver nanoparticles was found to be in the range of 7–8. The outlined procedure provides a rapid, facile and reproducible synthetic route to spherical AgNps of varying size and ensuing optical properties. Thus, this method is certain to find value in the many applications where size tunability of AgNps is desired.

 Please wait while we load your content...
Please wait while we load your content...