Characterization of the interaction between acotiamide hydrochloride and human serum albumin: 1H STD NMR spectroscopy, electrochemical measurement, and docking investigations†
Jiawei He,
Hongqin Yang,
Shanshan Li,
Kailin Xu,
Qing Wang,
Yanmei Huang and
Hui Li*
College of Chemical Engineering, Sichuan University, Chengdu, Sichuan, China. E-mail: lihuilab@sina.com; Fax: +86 028 85401207; Tel: +86 028 85405149
First published on 21st June 2016
Abstract
The interaction between acotiamide hydrochloride (Z-338) and human serum albumin (HSA) was investigated by multiple spectroscopic analyses, electrochemical approaches, and computer-aided molecular docking studies. 1H nuclear magnetic resonance (NMR) spectroscopy and saturation transfer difference (STD) data indicated that Z-338 weakly interacted with HSA. STD signals showed that the benzene and five-membered rings of Z-338 were responsible for the binding efficiency. Fluorescence lifetime measurements implied that Z-338 quenched the intrinsic fluorescence of HSA with a new complex formation via static mode. Key parameters regarding this interaction were calculated from differential pulse voltammetry and fluorescence spectroscopy. Results obtained from the two methods above ascertained the static mechanism and revealed that hydrogen bonding combined with van der Waals forces played a major role in HSA–Z-338 binding. Although the displacement of probes from both sites I and II was observed from competitive STD-NMR experiments, molecular docking results suggested that Z-338 was preferentially bound to site II of HSA. This finding was supported by the esterase-like activity result. A decrease in the esterase-like activity of HSA after Z-338 binding showed that the Arg-410 and Tyr-411 of subdomain IIIA were directly involved in the binding process, which corroborated the accuracy of docking studies. Furthermore, circular dichroism spectra, Fourier transform infrared spectroscopy, and 3D fluorescence demonstrated that Z-338 slightly disturbed the microenvironment of amino residues and affected the secondary structure of HSA. Overall, this study provides valuable information to further understand the use of Z-338.
1. Introduction
Acotiamide hydrochloride (N-[2-[bis(1-methylethyl) amino]ethyl]-2-[(2-hydroxy-4,5-dimethoxybenzoyl) amino] thiazole-4-carboxamide monohydrochloride trihydrate, Z-338) is a new drug currently being developed for the treatment of functional dyspepsia (FD).1 Several studies have demonstrated that Z-338 can inhibit the activity of acetylcholinesterase (AChE),2 which degrades the neurotransmitter acetylcholine (ACh). The beneficial effects of Z-338 on FD treatment in clinical studies have been proven across Europe, Japan, and the USA.3 As an AChE inhibitor, Z-338 is also used to treat myasthenia gravis.4Human serum albumin (HSA), as the most abundant carrier protein in the circulatory system, plays a major role in the transport and deposition of many endogenous and exogenous ligands.5,6 Given the availability of multiple interior hydrophobic pockets and the flexibility to adapt to the drug's shape,7 HSA can increase the apparent solubility of hydrophobic drugs and modulate drug delivery to cells in vivo. Therefore, the interaction between drugs and HSA has long been focused in many important research fields.8–10
Several methods have been employed to explore the binding of drugs to protein, such as equilibrium dialysis,11 isothermal titration calorimetry,12 capillary electrophoresis,13 and spectroscopic analysis.14 Another rising technique used for the system is nuclear magnetic resonance (NMR), which is a powerful tool for understanding weak binding processes at the molecular level and analyzing biological systems in real time under the systems' working conditions.15 Accordingly, the saturation transfer difference (STD) is an appropriate tool; it offers several advantages over normal methods to detect binding activity. First, the low-binding affinity ligands can usually be directly identified with high efficiency, even from a substance mixture. Second, the ligand possessing the strongest contact with the protein shows the most intense NMR signals, enabling the mapping of the ligand's binding epitope. And third, its high sensitivity allows using little protein with a molecular weight greater than 10 kDa. That is a crucial factor in NMR-based detection system.16–19 Given the advantages described above, STD-NMR methods have been widely used to characterize ligand binding in recent years.20–22 In the present study, the binding affinity of the H proton in Z-338 with HSA and possible binding site were explored by 1H NMR spectroscopy with the STD technique. To distinguish the quenching mechanism, fluorescence lifetime methods were utilized. The dominant binding parameters were obtained from differential pulse voltammetry (DPV) and fluorescence analyses. Molecular docking techniques were employed to visualize the main binding site of Z-338 on HSA, and the esterase-like activity of HSA after Z-338 binding was assessed. Moreover, the circular dichroism (CD), Fourier transform infrared spectroscopy (FT-IR), and 3D fluorescence spectra were obtained to analyze whether the secondary structure of HSA changed after Z-338 addition. Overall, this study will provide insights into the molecule-based interaction between Z-338 and HSA.
2. Materials and methods
2.1 Reagents and chemicals
Essential fatty acid free HSA was purchased from Sigma-Aldrich (Milwaukee, USA) and used without further purification. The stock solution of HSA (2.0 × 10−5 M) was prepared by dissolving solid HSA in 0.05 M PBS at pH 7.4 and stored at 0–4 °C. Acotiamide hydrochloride (Z-338) (≥99.8%) was purchased from Shandong RuiYue Biotechnology Co., Ltd. A stock solution of the drug (2.0 × 10−3 M) was prepared by dissolution in anhydrous ethanol. Warfarin and ibuprofen were supplied by Dalian Meilun Biology Technology Co., Ltd. (Dalian, China). Analytical-grade deuterium oxide (D2O, 99.9% purity), dimethyl sulfoxide-d6 (DMSO-d6), and p-nitrophenyl acetate (p-NPA) were obtained from J&K Scientific Ltd. (Beijing, China). All other solutions employed in this work were diluted to the required volume with PBS prepared from triple-distilled water, and all other reagents used were of analytical grade.2.2 NMR measurements
All NMR experiments were conducted on a Varian Inova 700 MHz spectrometer operating at 298 K by using VNMRJ software (version 2.1B). Stock solutions of Z-338 (4.0 × 10−2 M), warfarin (8.0 × 10−2 M), and ibuprofen (8.0 × 10−2 M) were prepared in DMSO-d6 and diluted with 0.05 M PBS (50% [v/v] D2O and H2O mixture) for further use. Sample volume was 600 μL in a 5 mm NMR tube. The final concentrations of HSA and Z-338 were 1.0 × 10−5 and 4.0 × 10−4 M (ratio of [HSA]/[drug] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40), respectively. Competition studies were performed using warfarin and ibuprofen, the two primary binding agents to sites I and II in HSA. Samples, which included identical HSA and Z-338 concentrations containing four different concentrations of warfarin (2.0 × 10−4 M, 4.0 × 10−4 M, 6.0 × 10−4 M and 8.0 × 10−4 M, respectively) or ibuprofen (2.0 × 10−4 M, 4.0 × 10−4 M, 6.0 × 10−4 M and 8.0 × 10−4 M, respectively), were analyzed. For the STD experiments, the selective saturation of HSA was achieved by a train of 50 ms Gaussian pulses. These pulses were applied at an on-resonance saturation of −0.5 ppm and an off-resonance saturation of −45 ppm. All the spectra were acquired using a sweep width of 8389.26 Hz, 256 transients, and acquisition time of 1 s. The total scan number in the STD experiments was 1024 with 16 ppm spectral widths in typical cases. Each sample was recorded with Watergate solvent suppression to obtain a standard 1H spectrum. All the spectra obtained were processed and analyzed using ACD/CNMR software (Advanced Chemistry Development, Inc., version 11.0).
40), respectively. Competition studies were performed using warfarin and ibuprofen, the two primary binding agents to sites I and II in HSA. Samples, which included identical HSA and Z-338 concentrations containing four different concentrations of warfarin (2.0 × 10−4 M, 4.0 × 10−4 M, 6.0 × 10−4 M and 8.0 × 10−4 M, respectively) or ibuprofen (2.0 × 10−4 M, 4.0 × 10−4 M, 6.0 × 10−4 M and 8.0 × 10−4 M, respectively), were analyzed. For the STD experiments, the selective saturation of HSA was achieved by a train of 50 ms Gaussian pulses. These pulses were applied at an on-resonance saturation of −0.5 ppm and an off-resonance saturation of −45 ppm. All the spectra were acquired using a sweep width of 8389.26 Hz, 256 transients, and acquisition time of 1 s. The total scan number in the STD experiments was 1024 with 16 ppm spectral widths in typical cases. Each sample was recorded with Watergate solvent suppression to obtain a standard 1H spectrum. All the spectra obtained were processed and analyzed using ACD/CNMR software (Advanced Chemistry Development, Inc., version 11.0).
2.3 Fluorescence spectroscopy
The fluorescence lifetimes of the HSA and HSA–Z-338 system were measured on a Horiba Jobin Yvon FluoroLog-TCSPC spectrofluorometer (HORIBA, France). The HSA concentration was fixed at 1.0 × 10−5 M, and the Z-338 concentration varied from 0 M to 2.0 × 10−5 M. Samples were recorded by fixing 280 nm as the excitation wavelength and 345 nm as the emission wavelength at room temperature. Experimental data were analyzed using the tail-fitting method, and the qualities of the fits were assessed by χ2 values and residuals.Fluorescence measurements were executed using a Cary Eclipse fluorescence spectrophotometer (Varian, USA) equipped with 1.0 cm quartz cells. Prior to the fluorescence measurements, an appropriate volume of Z-338 solution was added to HSA (2.0 × 10−6 M) that ranged from 0 M to 2.4 × 10−5 M and mixed for 30 min in a thermostat water bath at 298 K, 305 K, and 310 K. An excitation wavelength of 280 nm was employed, and the emission spectra were recorded from 300 nm to 500 nm, with widths of excitation and emission slits set at 5 and 10 nm, respectively. All fluorescence intensities were corrected to decrease the inner filter effect by using a previously reported method.23
The 3D fluorescence spectra of the HSA (2.0 × 10−6 M) and HSA–Z-338 system (molar ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) were obtained at an excitation wavelength range of 200–400 nm at 5 nm increments. The emission spectra were monitored from 200 nm to 500 nm.
10) were obtained at an excitation wavelength range of 200–400 nm at 5 nm increments. The emission spectra were monitored from 200 nm to 500 nm.
2.4 Electrochemical investigation
Electrochemical studies were performed on a CHI660E electrochemical workstation (Shanghai Chenhua Instruments Co., China), which was coupled with a conventional three-electrode cell composed of a platinum wire auxiliary electrode, a saturated calomel reference electrode, and a working electrode. The cyclic voltammetry (CV) and DPV behavioral patterns of Z-338 alone and after HSA addition in PBS at pH 7.4 were measured. After each addition of HSA to Z-338, an interaction time of 20 min was maintained, and a voltammogram was recorded. The CV measurements were recorded from −1.0 V to 1.0 V at a scan rate of 0.05 V s−1; the DPV was recorded from 0.2 V to 0.8 V with an amplitude of 50 mV and a pulse period of 0.5 s. The oxidative peak of Z-338 was selected for further analysis. To avoid the competitive adsorption between Z-338 and HSA at the electrode surface, a two-step procedure was developed as described in a ref. 24.2.5 Molecular docking
Docking studies were performed with the AutoDock 4.2 suite of programs, which utilizes the Lamarckian Genetic Algorithm (LGA) implemented therein. The native structure of HSA obtained from the Protein Data Bank (PDB ID: 1H9Z) was used as template. Receptor (HSA) and ligand (Z-338) files were prepared using AutoDock Tools. Grid maps of 126 × 126 × 126 points in the x, y, and z directions were calculated using AUTOGRID. During the calculation of grid maps, we applied a grid-point spacing of 0.896 Å. The LGA approach was employed to search the global optimum binding position. All the other parameters were in default settings. A subsequent round of dockings with the number of independent runs set to 150 was performed to locate the optimum binding site. The docked conformation with the lowest energy based on the AutoDock scoring function was selected as the binding mode. The output from AutoDock was rendered using Discovery Studio 3.1 software package (State Key Laboratory of Biotherapy, Sichuan University, China).2.6 Esterase activity assay
The effect of Z-338 on the esterase activity of HSA toward p-NPA was analyzed on a TU-1901 UV-vis spectrophotometer (Persee, Beijing, China) by steady-state kinetics at 298 K. The concentration of HSA was kept constant at 1.2 × 10−5 M, whereas the concentration of p-NPA was varied from 0 M to 7.0 × 10−4 M. The rate of p-NPA hydrolysis was determined by measuring the appearance of p-nitrophenol, a yellow product, at 405 nm for 60 s.25 The concentration of p-nitrophenol was determined from the observed absorbance and a standard curve (y = 0.01274x + 0.00444; R = 0.9999).2.7 CD spectroscopy
The CD spectra of HSA in the absence and presence of Z-338 were measured using Jasco J-815 Circular Dichroism Spectropolarimeter (Jasco, Japan) at 298 K. The spectra of both HSA and the HSA–Z-338 complex with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 were recorded in the range of 200–250 nm. Data were collected with an interval of 1 nm and a scan speed of 100 nm min−1 at 298 K. The corresponding buffer solution was used for baseline correction. All the reported spectra were the average of three consecutive scans for each sample.
10 were recorded in the range of 200–250 nm. Data were collected with an interval of 1 nm and a scan speed of 100 nm min−1 at 298 K. The corresponding buffer solution was used for baseline correction. All the reported spectra were the average of three consecutive scans for each sample.
2.8 FT-IR spectroscopy
FT-IR measurements were conducted on a Nicolet-6700 FT-IR (Thermo, USA) spectrometer with a smart OMNI-sampler accessory. The infrared (IR) spectra were obtained using the ATR method at a resolution of 4 cm−1 and with 64 scans at room temperature. The absorption spectra of both the HSA and HSA–Z-338 complexes with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were recorded in the range of 4000–600 cm−1. The corresponding absorbance contributions of the PBS and free Z-338 solutions were recorded and digitally subtracted from the same instrumental parameters.
1 were recorded in the range of 4000–600 cm−1. The corresponding absorbance contributions of the PBS and free Z-338 solutions were recorded and digitally subtracted from the same instrumental parameters.
3. Results and discussion
3.1 NMR spectroscopy for HSA–Z-338 system
NMR technique, as a well-established powerful method, is extensively used to study the interaction between small molecular compounds and biological macromolecules, particularly for screening applications in the pharmaceutical industry.26 The ligand-observed screening is attractive specifically because of its few limitations, such as protein size, non-requirement for isotope labeling, and performance under low protein concentrations.27 In recent years, STD-NMR spectroscopy, as a new NMR-based screening method, was applied to characterize the drug–protein binding activity and has attracted considerable interest among researchers.21,28 We report herein the binding interaction between Z-338 and HSA, based on 1H NMR spectroscopy using STD technique. This technique exploited the nuclear Overhauser effect (NOE) transference from the macromolecule to the ligand, under physiological conditions of temperature and pH, and without labeling requirements. When a macromolecule was saturated by a selective radio-frequency irradiation, this saturation will spread within the entire macromolecule via spin diffusion, and will subsequently be transferred to the bound ligand through intermolecular NOEs.26 The most tightly bound ligand 1H with the macromolecule will receive the most intense magnetization transfer and provide the most intense NMR signals.27Fig. 1 displays the reference 1H NMR spectrum Z-338 with HSA in a 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (a) and the corresponding STD-NMR spectrum (b). The full STD-NMR spectrum of HSA–Z-338 system is depicted in the ESI as Fig. S1.† As shown in Fig. 1, similar signals of Z-338 can be observed between the 1H NMR and STD-NMR spectra. Protons of the Z-338 molecule nearest to the HSA can easily be identified from the STD spectrum because these components are saturated to the highest degree.29 The STD effects on the H-5, H-20, H-27, H-16, H-10, and H-13 of the Z-338 molecule were observed despite the weak STD signals. Therefore, the benzene and five-membered rings of Z-338 may assume the most intimate contact with HSA. This phenomenon proved that the Z-338 bound to the HSA receptor slightly, and the 1H STD-NMR technique can effectively detect the binding process between Z-338 and HSA. Detailed information on the weak interaction was discussed as follows.
1 ratio (a) and the corresponding STD-NMR spectrum (b). The full STD-NMR spectrum of HSA–Z-338 system is depicted in the ESI as Fig. S1.† As shown in Fig. 1, similar signals of Z-338 can be observed between the 1H NMR and STD-NMR spectra. Protons of the Z-338 molecule nearest to the HSA can easily be identified from the STD spectrum because these components are saturated to the highest degree.29 The STD effects on the H-5, H-20, H-27, H-16, H-10, and H-13 of the Z-338 molecule were observed despite the weak STD signals. Therefore, the benzene and five-membered rings of Z-338 may assume the most intimate contact with HSA. This phenomenon proved that the Z-338 bound to the HSA receptor slightly, and the 1H STD-NMR technique can effectively detect the binding process between Z-338 and HSA. Detailed information on the weak interaction was discussed as follows.
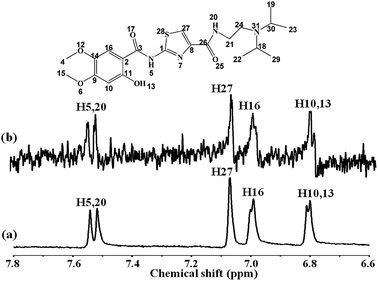 | ||
Fig. 1 1H NMR spectrum of Z-338 and HSA in 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio obtained with a Watergate scheme for solvent suppression (a) and the corresponding STD spectrum (b). 1 ratio obtained with a Watergate scheme for solvent suppression (a) and the corresponding STD spectrum (b). | ||
3.2 Analysis of fluorescence quenching of HSA by Z-338
Fluorescence techniques were used to provide information on the binding properties of small molecules to a protein.30 In the present work, the interaction between Z-338 and HSA was investigated by measuring the intrinsic fluorescence intensity of HSA in the absence and presence of Z-338. The fluorescence spectra are illustrated in Fig. 2. A significant decrease in the fluorescence intensity of HSA was observed when the Z-338 was titrated into a fixed concentration of HSA. Generally, the intrinsic fluorescence of HSA originates from the tryptophan (Trp), tyrosine (Tyr), and phenylalanine (Phe) residues, with the last residue contributing only slightly.31 Some small molecules can change the microenvironment of a fluorophore and decrease the intrinsic fluorescence intensity of HSA by interaction. Thus, the observed decrease in fluorescence intensity can be ascribed to the binding of Z-338 with HSA, which confirmed the 1H NMR results.A decrease in fluorescence intensity is known as quenching.32 Fluorescence quenching can generally be classified as static when caused by ground-state complex formation and dynamic when arising from diffusion. The quenching mechanisms can be distinguished by the differences in temperature-dependent behavior and calculated by the well-known Stern–Volmer equation.33
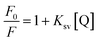 | (1) |
To further distinguish static quenching from dynamic quenching, we measured the fluorescence lifetimes of HSA and the HSA–Z-338 system. The average fluorescence lifetime (〈τ〉) was calculated from the decay times and the relative amplitude (α) by using the following relation:34
| 〈τ〉 = τ1α1 + τ2α2 | (2) |
The fluorescence decay of HSA in the absence and presence of Z-338 is depicted in Fig. 2 (inset, a–c) and Table 1. Fig. 2 and Table 1 reveal that the fluorescence decay curve of HSA is a single exponential with a lifetime value of 5.689 ns. After Z-338 addition, the average lifetime of the HSA fluorophore marginally decreased to 5.514 ns. Generally, static quenching with the formation of static ground-state complexes does not decrease the decay time of the uncomplexed fluorophores.35 A decrease in the mean decay time of the entire excited-state population should be attributed to dynamic quenching, which is a rate process acting on the entire excited-state population. The bound Z-338 may directly influence the fluorophore lifetime, and the observed fluorescence originates from the uncomplexed fluorophores. A minimal decrease in the average lifetime of uncomplexed fluorophores was noted with increasing Z-338 concentration. This alteration of the average fluorescence lifetime in the tested systems suggests that a new complex (HSA–Z-338) was formed by the static quenching process, a result consistent with the fluorescence quenching analysis findings. By forming a complex with the HSA, the Z-338 molecule can be temporarily stored in the plasma and employed to prolong the action time.
| System | CZ-338 (μM) | τ1 (ns) | τ2 (ns) | α1 | α2 | 〈τ〉 (ns) | χ2 |
|---|---|---|---|---|---|---|---|
| HSA–Z-338 | 0 | 2.451 | 6.633 | 0.226 | 0.774 | 5.689 | 0.993 |
| 10 | 2.510 | 6.644 | 0.239 | 0.761 | 5.656 | 1.049 | |
| 20 | 2.047 | 6.326 | 0.190 | 0.810 | 5.514 | 1.033 |
3.3 Binding constant and number of binding sites
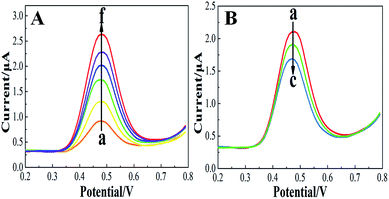
Considering that the quenching mechanism is static, we determined the binding constant and the number of binding sites by electrochemical approaches and fluorescence spectroscopy. Fig. S2† shows the CV curves of Z-338 bound and unbound to HSA. As shown in Fig. S2,† an oxidative peak appeared close to 0.5 V. This peak has been assigned to the hydroxyl in the benzene ring of the Z-338 molecule. After HSA was added, the oxidative peak slightly decreased. Such phenomenon might be attributed to the formation of an electrochemical non-active complex.36 To further analyze the binding property, DPV was conducted. Fig. 3A presents the DPV curves of Z-338 at different concentrations without HSA. Fig. 3B displays the DPV of Z-338 (0.5 × 10−3 M) in the absence (curve a) and presence of HSA (curves b and c). As shown in Fig. 3, the oxidative peak increased with increasing concentration of Z-338. However, the oxidative peak of Z-338 decreased when HSA was present. These results confirmed the binding process between Z-338 and HSA. To quantitatively analyze the DPV data, we determined the binding constant β and the number of binding sites m by using the following equation:37
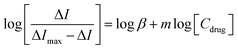 | (3) |
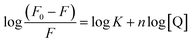 | (4) |
From the equations above, the binding constant β (values obtained from the DPV method) and K (present values obtained from fluorescence analysis) at three temperatures are summarized in Table 2. The number of bound sites m/n was also calculated. Where m = 1.429, 1.446, and 1.496 at 298 K, 305 K, and 310 K, respectively and n = 0.991, 0.987, and 0.954 at 298 K, 305 K, and 310 K respectively. The binding parameters obtained by the two approaches above were consistent.
| System | T/K | βa(Kb) (104 M−1) | ΔGa(ΔGb) (kJ mol−1) | ΔHa(ΔHb) (kJ mol−1) | ΔSa(ΔSb) (J mol−1 K−1) |
|---|---|---|---|---|---|
| a The binding constants and thermodynamic parameters obtained from differential pulse voltammetry analysis.b Binding constants and thermodynamic parameters obtained from fluorescence analysis. The values within parentheses were gotten from fluorescence method. | |||||
| HSA–Z-338 | 298 | 2.606(2.985) | −25.18(−25.61) | ||
| 305 | 1.337(1.888) | −24.19(−24.76) | −67.42(−61.56) | −141.75(−120.64) | |
| 310 | 0.914(1.125) | −23.48(−24.16) | |||
The β/K values decreased with increasing temperatures, further confirming that the quenching was static. Compared with other strong protein–drug systems with binding constants over 106 M−1, HSA–Z-338 exhibited more moderate binding constants. This conclusion was in line with the STD-NMR results indicating that the binding affinity between HSA and Z-338 was weak. The weak affinity between Z-338 and HSA may promote the diffusion of Z-338 from the circulatory system to the target organ (stomach).38 In addition, the number of binding sites m/n was approximately close to one. Therefore, at least one binding site with high affinity of Z-338 in HSA was present.
3.4 Thermodynamic analysis and the nature of binding forces
Given the binding constants of different temperatures, thermodynamic parameters such as ΔG, ΔH, and ΔS can be obtained using the van't Hoff equation and Gibbs–Helmholtz equation.39 The nature of binding forces can be well explained by these parameters. The negative values for ΔG in Table 2 indicate that the binding process of HSA with Z-338 occurred spontaneously. Four acting forces, namely, hydrogen bonds, van der Waals forces, electrostatic forces, and hydrophobic interaction forces, usually exist between small molecules and macromolecules. In Table 2, the negative values of ΔH and ΔS denote that both hydrogen bonds and van der Waals forces contributed dominantly in the formation and stabilization of the HSA–Z-338 complex.403.5 Identification of the HSA main binding site
To identify the binding sites of Z-338 on HSA, we regarded competitive STD-NMR experiments as a potential way of comparing the relative affinities of the competing compounds (Z-338 competed with warfarin/ibuprofen and the ratio of [Z-338]/[warfarin/ibuprofen] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) for HSA. The site marker was titrated in a solution containing fixed amounts of Z-338 and HSA. The competitive STD-NMR spectra are presented in Fig. 4. As shown in Fig. 4, the signals of Z-338 were completely abolished after the two site markers added. To further obtain the accurate information about the binding sites, we conducted another STD competition studies by altering the concentration of site markers to confirm whether Z-338 and the site markers compete for the same binding site on the protein or not. The results are depicted in the ESI as Fig. S3 and S4.† By close observation of these spectra, we can draw the conclusion that Z-338 competed with warfarin/ibuprofen in both sites I and II. That is, Z-338 can bind with HSA in site I or site II.45 In addition, molecular docking studies were conducted to determine the preferable binding site and obtain a detailed structural representation.
2) for HSA. The site marker was titrated in a solution containing fixed amounts of Z-338 and HSA. The competitive STD-NMR spectra are presented in Fig. 4. As shown in Fig. 4, the signals of Z-338 were completely abolished after the two site markers added. To further obtain the accurate information about the binding sites, we conducted another STD competition studies by altering the concentration of site markers to confirm whether Z-338 and the site markers compete for the same binding site on the protein or not. The results are depicted in the ESI as Fig. S3 and S4.† By close observation of these spectra, we can draw the conclusion that Z-338 competed with warfarin/ibuprofen in both sites I and II. That is, Z-338 can bind with HSA in site I or site II.45 In addition, molecular docking studies were conducted to determine the preferable binding site and obtain a detailed structural representation.
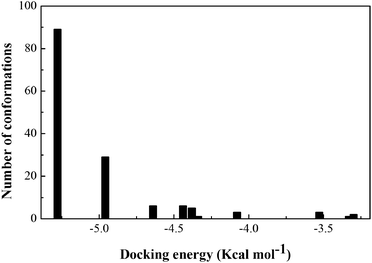
The stereo view of the favorable docked configuration (Fig. 6A) displays that the Z-338 molecule is well inserted into the hydrophobic cavity of subdomain IIIA, i.e., drug site II of HSA. The predicted binding model results indicated that Z-338 is a higher affinity site II binder. In addition, the optimum interaction between Z-338 and HSA is found to be characterized by a favorable binding energy of −5.28 kcal mol−1, with an inhibition constant of 7.06 × 10−5 M. At this juncture, the docking simulation results are necessary for comparison with those obtained from the experiments, i.e., the results from DPV and fluorescence methods. For this purpose, we adopted the assumption that the predicted binding constant is the reciprocal of the inhibition constant acquired from docking analysis. With this assumption, the binding constant of the HSA–Z-338 interaction predicted from the docking result was found to be 1.42 × 104 M−1. The free energy change can be calculated from ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K, that is, −23.69 kJ mol−1 at 298 K. These above findings revealed by the employed blind-docking simulation are in line with experimental results (Sections 3.3 and 3.4), establishing the reliability of the findings, and may substantiate the practical applicability and feasibility of the methods employed to estimate the parameters.
K, that is, −23.69 kJ mol−1 at 298 K. These above findings revealed by the employed blind-docking simulation are in line with experimental results (Sections 3.3 and 3.4), establishing the reliability of the findings, and may substantiate the practical applicability and feasibility of the methods employed to estimate the parameters.
To better visualize the binding mode, the 2D ligand interaction diagram is generated and shown in Fig. 6B. The Z-338 molecule is mainly surrounded by Asn-391, Cys-392, Cys-438, Ile-388, Leu-457, Arg-485, Ser-489, Phe-488, Val-473, Val-426, Leu-491, Val-415, Arg-410, Leu-460, Tyr-411, Leu-453, Leu-430, Leu-407, Gly-431, and Phe-403, which are active amino acids for the HSA–ligand interaction. Two hydrogen bonds were found to exist in the HSA–Z-338 system. The hydroxyl in the benzene ring of the Z-338 molecule came in contact with Leu-430 through one hydrogen bond with a distance of 2.094 Å. Tyr-411 was in a suitable position to form another hydrogen bond with the O atom in carbonyl at 2.159 Å. The newly formed hydrogen bonds may make the Z-338 ligand adapt to the shape of a pocket in subdomain IIIA more easily, thereby stabilizing the docking conformation. In addition, when the ligand entered into the receptor, both molecules underwent changes to minimize the space between the two molecules under the influence of van der Waals forces.47 The term “van der Waals forces” is generally used to denote a variety of nonspecific interactions, such as dipole/dipole, multipole/multipole, induced dipole/induced dipole, and even cation/dipole interactions. However, recent studies attempted to reveal the details underlying these distinct interactions.48 For instance, the interactions involving π electron systems have been shown to include cations and CH groups. Cation/π interactions usually occur between a positively charged molecule and a π system, i.e., aromatic rings, alkenes, and alkynes. Fig. 6B shows that cation/π interactions occur within the HSA–Z-338 system. The residue Arg-410 acts as a cation participating in the cation/π interactions with two rings (benzene and five-membered rings). In conclusion, this docking conformation reconfirmed that the binding forces were mainly governed by hydrogen bonds and van der Waals forces.
 | (5) |
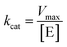 | (6) |
The effect of Z-338 binding on the esterase-like activity of HSA was analyzed by monitoring the rate of p-NPA hydrolysis as described above at different Z-338![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HSA ratios (0
HSA ratios (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.25
1, 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.50
1, 0.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.75
1, 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). By measuring the concentration of p-nitrophenol, the rate of p-NPA hydrolysis was determined. The values of kcat and Km are presented in Table 3. The Km values for the hydrolysis of p-NPA by HSA clearly increased from 58.27 μM to 127.31 μM, whereas the kcat values were similar at different HSA
1). By measuring the concentration of p-nitrophenol, the rate of p-NPA hydrolysis was determined. The values of kcat and Km are presented in Table 3. The Km values for the hydrolysis of p-NPA by HSA clearly increased from 58.27 μM to 127.31 μM, whereas the kcat values were similar at different HSA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z-338 molar ratios. Ultimately, the progressively increased Km values and similar kcat values diminished the catalytic efficiency (kcat/Km) of HSA toward p-NPA with increasing Z-338 concentrations. Furthermore, the kinetic data were plotted as Lineweaver–Burk plots (Fig. 7). The increased slope with constant intercept at a series of Z-338 concentrations indicated competitive inhibition of HSA's esterase-like activity by Z-338. On the basis of the above results, we conclude that Z-338 inhibited the esterase-like activity of HSA by binding to the active site residues Arg-410 and Tyr 411 at subdomain IIIA of HSA, which corroborates the accuracy of the docking results. In particular, Z-338 binding to site II was confirmed.
Z-338 molar ratios. Ultimately, the progressively increased Km values and similar kcat values diminished the catalytic efficiency (kcat/Km) of HSA toward p-NPA with increasing Z-338 concentrations. Furthermore, the kinetic data were plotted as Lineweaver–Burk plots (Fig. 7). The increased slope with constant intercept at a series of Z-338 concentrations indicated competitive inhibition of HSA's esterase-like activity by Z-338. On the basis of the above results, we conclude that Z-338 inhibited the esterase-like activity of HSA by binding to the active site residues Arg-410 and Tyr 411 at subdomain IIIA of HSA, which corroborates the accuracy of the docking results. In particular, Z-338 binding to site II was confirmed.
HSA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) drug drug |
Km (μM) | kcat (s−1) | kcat/Km (M−1 s−1) |
|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
58.27 | 0.0092 | 157.89 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
75.69 | 0.0094 | 124.19 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50 0.50 |
92.32 | 0.0096 | 103.99 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
105.85 | 0.0097 | 91.64 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
127.31 | 0.0101 | 79.33 |
3.6 Conformational investigations
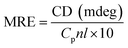 | (7) |
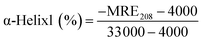 | (8) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 are the MRE values of β-form with random coil conformation and a pure α-helix at 208 nm, respectively.
000 are the MRE values of β-form with random coil conformation and a pure α-helix at 208 nm, respectively.
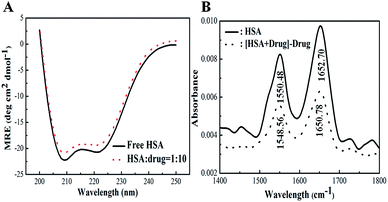
By applying the preceding equations, we noted that the percentage of α-helices in free HSA (∼63.8%) agrees closely with literature reports.41 A loss in the α-helical content from 63.8% to 59.6% after binding with 2.0 × 10−5 M Z-338 suggested minor peptide strand unfolding, thus apparently demonstrating a drug-induced perturbation of the secondary structure of HSA. This binding process may partially destroy the original hydrogen bonding network of HSA, thus making the polypeptide chain become tender to accommodate the Z-338 molecule in a specific manner inside the protein. Hence, this minor decrease in α-helical content may not affect the physiological function of this carrier protein during the transport of the Z-338 molecule.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations) and amide II (1500–1600 cm−1, C–N stretch coupled with N–H bending modes) bands, which are sensitive to protein secondary structure, were investigated frequently.53
O stretching vibrations) and amide II (1500–1600 cm−1, C–N stretch coupled with N–H bending modes) bands, which are sensitive to protein secondary structure, were investigated frequently.53As shown in Fig. 8B, the FT-IR spectra of free and Z-338-bound forms of HSA were obtained. The characteristic amide I and II absorption peak positions of free HSA were at 1652.70 and 1550.48 cm−1, correspondingly. After Z-338 addition, the two amide bands apparently decreased with a peak shift (amide I: from 1652.70 cm−1 to 1650.78 cm−1; amide II: from 1550.48 cm−1 to 1548.56 cm−1) in absorbance intensity. The peak position shift of amide I, along with the decrease in peak intensity, implies that the α-helical content of HSA has been changed. All these changes demonstrated that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N groups in the protein polypeptides were perturbed, leading to conformational changes in the secondary structure of HSA.
O and C–N groups in the protein polypeptides were perturbed, leading to conformational changes in the secondary structure of HSA.
 | ||
Fig. 9 3D fluorescence spectra of HSA alone (2.0 × 10−6 M) and in the presence of Z-338 ([HSA]/[Z-338] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 10). | ||
| System | Peak no. | Peak position [λex/λem (nm nm−1)] | Intensity F |
|---|---|---|---|
| HSA | a | 280/280 → 350/350 | 230.48 → 372.87 |
| I | 280/338 | 565.19 | |
| II | 225/338 | 543.28 | |
| HSA–Z-338 | a | 280/280 → 350/350 | 246.58 → 408.20 |
| I | 280/342 | 472.71 | |
| II | 225/344 | 337.74 |
Peak a, which refers to the Rayleigh scattering peak (λex = λem), increased with the addition of Z-338.55 This phenomenon may be attributed to the formation of HSA–Z-338 complexes, which induced the change in macromolecular diameter and enhanced the intensity of peak a. Peak I refers to the spectral characteristic of the Trp and Tyr residues, and peak II reveals the fluorescence behavior of the polypeptide backbone structures.56 After Z-338 addition, the HSA solution produced a decrease in intensity along with a slight red shift of the maximum emission wavelength in peaks I and II. Table 4 shows that the peak I of the HSA–Z-338 complex decreased by 16% along with a minor red shift of 4 nm. Peak II of the HSA–Z-338 complex red shifted by 6 nm with an 38% decrease in intensity. Hydrogen bonding is indispensable in the formation of HSA secondary and tertiary structures because the structures and functions of several biological molecules are highly dependent on intramolecular hydrogen bonding. From the analysis of intensity changes and peak shifts, we conclude that Z-338 presents minimal effects on the conformation of HSA. As such, the formed hydrogen bond between Z-338 and HSA does not show significant influences on the original bonding, and this condition may be beneficial for HSA.
4. Conclusions
This work first demonstrated a detailed binding mechanism of Z-338 with HSA. The protons in benzene and five-membered rings of Z-338 bound tightly with the hydrophobic cavity of HSA in site II. Hydrogen bonding and van der Waals forces were the main elements to drive the HSA–Z-338 binding reaction. This interaction did not affect the stability of HSA, though the newly formed hydrogen bonds disturbed the original H-network of this protein. However, the esterase-like activity of HSA decreased and the secondary structure of HSA changed after Z-338 addition. Namely, the binding of Z-338 on HSA influenced the catalytic activity and conformation of the latter biomolecule. STD-NMR techniques employed in our study provided the binding affinity of H proton in Z-338 with HSA and ascertained the binding site. The static quenching mechanism was confirmed by fluorescence lifetime measurements. The results obtained from the experiments were in line with molecular docking simulation. Overall, this study can provide some important biophysical insights into the binding mechanisms between drugs and proteins.Acknowledgements
This work was supported by the Applied Basic Research Project of Sichuan Province (Grant No. 2014JY0042), the National Development and Reform Commission and Education of China (Grant No. 2014BW011) and the Large-scale Science Instrument Shareable Platform Construction of Sichuan Province (Grant No. 2015JCPT0005-15010102).References
- K. Seto, T. Sasaki, K. Katsunuma, N. Kobayashi, K. Tanaka and J. Tack, Neurogastroenterol. Motil., 2008, 20, 1051–1059 CrossRef CAS PubMed.
- Y. Matsunaga, T. Tanaka, K. Yoshinaga, S. Ueki, Y. Hori, R. Eta, Y. Kawabata, K. Yoshii, K. Yoshida and T. Matsumura, J. Pharmacol. Exp. Ther., 2011, 336, 791–800 CrossRef CAS PubMed.
- E. Altan, T. Masaoka, R. Farré and J. Tack, Expert Rev. Gastroenterol. Hepatol., 2012, 6, 533–544 CrossRef CAS PubMed.
- F. Romi, N. Gilhus and J. Aarli, Acta Neurol. Scand., 2005, 111, 134–141 CrossRef CAS PubMed.
- A. Robertson and R. Brodersen, Dev. Pharmacol. Ther., 1990, 17, 95–99 CrossRef.
- H. Vorum and B. Honoré, J. Pharm. Pharmacol., 1996, 48, 870–875 CrossRef CAS PubMed.
- A. Barik, B. Mishra, A. Kunwar and K. I. Priyadarsini, Chem. Phys. Lett., 2007, 436, 239–243 CrossRef CAS.
- D. C. Carter, X.-M. He, S. H. Munson, P. D. Twigg, K. M. Gernert, M. B. Broom and T. Y. Miller, Science, 1989, 244, 1195–1198 CrossRef CAS PubMed.
- N. Ramachandran, E. Hainsworth, B. Bhullar, S. Eisenstein, B. Rosen, A. Y. Lau, J. C. Walter and J. LaBaer, Science, 2004, 305, 86–90 CrossRef CAS PubMed.
- J. Li and S. Wang, J. Chem. Thermodyn., 2013, 58, 206–210 CrossRef CAS.
- M. H. Rahman, T. Maruyama, T. Okada, K. Yamasaki and M. Otagiri, Biochem. Pharmacol., 1993, 46, 1721–1731 CrossRef CAS PubMed.
- A. Saboury, J. Iran. Chem. Soc., 2006, 3, 1–21 CrossRef CAS.
- N. Sisavath, L. Leclercq, T. Le Saux, F. Oukacine and H. Cottet, J. Chromatogr. A, 2013, 1289, 127–132 CrossRef CAS PubMed.
- R. Thakur, A. Das and A. Chakraborty, RSC Adv., 2014, 4, 14335–14347 RSC.
- A. Jana, K. T. Nguyen, X. Li, P. Zhu, N. S. Tan, H. Ågren and Y. Zhao, ACS Nano, 2014, 8, 5939–5952 CrossRef CAS PubMed.
- M. Mayer and B. Meyer, Angew. Chem., Int. Ed., 1999, 38, 1784–1788 CrossRef CAS.
- J. Klein, R. Meinecke, M. Mayer and B. Meyer, J. Am. Chem. Soc., 1999, 121, 5336–5337 CrossRef CAS.
- K. Umemoto, S. Oikawa, M. Aida and Y. Sugawara, J. Biomol. Struct. Dyn., 1988, 6, 593–608 Search PubMed.
- H. Maaheimo, P. Kosma, L. Brade, H. Brade and T. Peters, Biochemistry, 2000, 39, 12778–12788 CrossRef CAS PubMed.
- C. D. Milagre, L. F. Cabeça, W. P. Almeida and A. J. Marsaioli, J. Braz. Chem. Soc., 2012, 23, 403–408 CAS.
- D. M. Dias, J. P. Rodrigues, N. S. Domingues, A. M. Bonvin and M. Castro, Eur. J. Inorg. Chem., 2013, 2013, 4619–4627 CrossRef CAS.
- H. Yang, Y. Huang, D. Wu, J. Yan, J. He and H. Li, New J. Chem., 2016, 40, 2530–2540 RSC.
- Y. Cahyana and M. H. Gordon, Food Chem., 2013, 141, 2278–2285 CrossRef CAS PubMed.
- J. Yang, Y. Dong, L. Wang, D. Zhang, Z. Zhang and L. Zhang, Sens. Actuators, B, 2015, 211, 59–66 CrossRef CAS.
- M. T. Rehman, H. Shamsi and A. U. Khan, Mol. Pharm., 2014, 11, 1785–1797 CrossRef CAS PubMed.
- M. Mayer and B. Meyer, J. Am. Chem. Soc., 2001, 123, 6108–6117 CrossRef CAS PubMed.
- X. Zhou, X. Li and X. Chen, Dyes Pigm., 2013, 98, 212–220 CrossRef CAS.
- A. Sivertsen, J. Isaksson, H.-K. S. Leiros, J. Svenson, J.-S. Svendsen and B. O. Brandsdal, BMC Struct. Biol., 2014, 14, 1 CrossRef PubMed.
- C. Cruz, R. E. Boto, P. Almeida and J. A. Queiroz, J. Mol. Recognit., 2011, 24, 975–980 CrossRef PubMed.
- P. Kandagal, J. Seetharamappa, S. Ashoka, S. Shaikh and D. Manjunatha, Int. J. Biol. Macromol., 2006, 39, 234–239 CrossRef CAS PubMed.
- N. V. Rakotoarivelo, P. Perio, E. Najahi and F. o. Nepveu, J. Phys. Chem. B, 2014, 118, 13477–13485 CrossRef CAS PubMed.
- J. R. Lakowicz and B. R. Masters, J. Biomed. Opt., 2008, 13, 9901 CrossRef.
- B. Valeur and M. N. Berberan-Santos, Molecular fluorescence: principles and applications, John Wiley & Sons, 2012 Search PubMed.
- P. Mandal, M. Bardhan and T. Ganguly, J. Photochem. Photobiol., B, 2010, 99, 78–86 CrossRef CAS PubMed.
- G. Paramaguru, A. Kathiravan, S. Selvaraj, P. Venuvanalingam and R. Renganathan, J. Hazard. Mater., 2010, 175, 985–991 CrossRef CAS PubMed.
- Z.-Q. Xu, B. Zhou, F.-L. Jiang, J. Dai and Y. Liu, Colloids Surf., B, 2013, 110, 321–326 CrossRef CAS PubMed.
- Y. Wu, X. Ji and S. Hu, Bioelectrochemistry, 2004, 64, 91–97 CrossRef CAS PubMed.
- N. Shahabadi, S. Hadidi and F. Feizi, Spectrochim. Acta, Part A, 2015, 138, 169–175 CrossRef CAS PubMed.
- D. T. Haynie, Biological thermodynamics, Cambridge University Press, 2001 Search PubMed.
- P. D. Ross and S. Subramanian, Biochemistry, 1981, 20, 3096–3102 CrossRef CAS PubMed.
- D. C. Carter and J. X. Ho, Adv. Protein Chem., 1994, 45, 153–203 CrossRef CAS PubMed.
- G. Sudlow, D. J. Birkett and D. N. Wade, Clin. Exp. Pharmacol. Physiol., 1975, 2, 129–140 CrossRef CAS PubMed.
- G. Sudlow, D. Birkett and D. Wade, Mol. Pharmacol., 1976, 12, 1052–1061 CAS.
- U. K. Hansen, L. Minchiotti, S. O. Brenan and O. Sugita, Eur. J. Biochem., 1990, 193, 169–174 CrossRef.
- S. A. Tanoli, N. U. Tanoli, T. M. Bondancia, S. Usmani, Z. Ul-Haq, J. B. Fernandes, S. S. Thomasi and A. G. Ferreira, RSC Adv., 2015, 5, 23431–23442 RSC.
- M. Sarkar, S. S. Paul and K. K. Mukherjea, J. Lumin., 2013, 142, 220–230 CrossRef CAS.
- G. U. Nienhaus, Protein–ligand interactions: methods and applications, Humana Press, Totowa, NJ, 2005 Search PubMed.
- A. C. A. Roque, Ligand-Macromolecular Interactions in Drug Discovery, Springer, 2010 Search PubMed.
- G. E. Means and M. L. Bender, Biochemistry, 1975, 14, 4989–4994 CrossRef CAS PubMed.
- G. Means and H.-L. Wu, Arch. Biochem. Biophys., 1979, 194, 526–530 CrossRef CAS PubMed.
- H. Watanabe, S. Tanase, K. Nakajou, T. Maruyama, U. Kragh-Hansen and M. Otagiri, Biochem. J., 2000, 349, 813–819 CrossRef CAS PubMed.
- U. Anand, L. Kurup and S. Mukherjee, Phys. Chem. Chem. Phys., 2012, 14, 4250–4258 RSC.
- H.-X. Zhang, X. Huang, P. Mei, K.-H. Li and C.-N. Yan, J. Fluoresc., 2006, 16, 287–294 CrossRef CAS PubMed.
- D. Wu, J. Yan, P. Tang, S. Li, K. Xu and H. Li, Food Chem., 2015, 188, 370–376 CrossRef CAS PubMed.
- M. S. Zaroog and S. Tayyab, Process Biochem., 2012, 47, 775–784 CrossRef CAS.
- D. Li, T. Zhang, C. Xu and B. Ji, J. Photochem. Photobiol., B, 2011, 104, 414–424 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra08310b |
| This journal is © The Royal Society of Chemistry 2016 |