An eco-friendly physicocultural-based rapid synthesis of selenium nanoparticles
Shubhangi Shirsat*a,
Ambadas Kadamb,
Vijaykumar V. Jadhavc,
Manohar K. Zated,
Mu. Naushade,
B. N. Pawarf,
Rajaram S. Mane*deg and
Kwang Ho Kim*cg
aDepartment of Biotechnology, New Model Degree College, Hingoli, M.S., India. E-mail: biotechshubhangi@yahoo.com
bDepartment of Botany, DSM'S ACS, College, Jintur, Dist., Parbhani, M.S., India. E-mail: kadamambadas@rediffmail.com
cSchool of Material Sciences and Engineering, Pusan National University, Busan 609-735, Republic of Korea. E-mail: kwhokim@pusan.ac.kr
dSchool of Physical Sciences, SRTM University, Nanded, M.S., India. E-mail: rsmane_2000@yahoo.com
eDepartment of Chemistry, College of Science, King Saud University, Bld#5, Riyadh, Saudi Arabia
fDepartment of Physics, Bharati Vidyapeeth University, Yashwantrao Mohite College, Pune 38, India
gGlobal Frontier R & D Center for Hybrid Interface Materials, Pusan National University, 30 Jangjeon-dong, Geumjung-gu, Busan 609-735, Republic of Korea
First published on 5th May 2016
Abstract
The eco-friendly, safe, and cost-effective synthesis of selenium (Se) nanoparticles (NPs) by microorganisms is an important branch of nanotechnology and is considered to be a green synthesis. Functional biosynthesized Se NPs find various applications if they are have definite shapes and sizes, which can be obtained by controlling the cultural conditions and physical operation parameters during the biosynthesis process. We depict the rapid (∼12 h) synthesis of Se NPs using a tryptic soy broth culture under the experimental conditions of pH ∼9, temperature ∼50 °C, in sunlight. The optimized conditions helped us to synthesize chemically and environmentally stable Se NPs. The main aim of the present study is to optimize the different cultural media, temperature, pH, light intensity, concentration of selenium dioxide, and the time of reaction for the rapid blend of Se NPs by Pantoea agglomerans.
1. Introduction
The concepts of biological, physical, material, and chemical sciences are merging together in nanotechnology for the development of a variety of technologies. Particles with sizes in the range of 100 nm or less, which are referred to as nanoparticles (NPs), have 1D–3D dimensions and are a structural and functional parts of nanotechnology.1 Currently, extra attention is being paid to nanomaterials as they have unusual properties as compared to their polycrystalline counterparts.2,3 Selenium (Se), one of the chalcogenides, which has many applications in the field of electronics, such as in rectifiers, solar cells, photography, and xerography as well as applications in the glass industry to remove the unwanted green tone caused by iron oxide impurities,4,5 occurs in various forms including selenate SeO42−, selenite SeO32−, selenide Se2−, etc. On the other hand, from a life-sciences point of view, Se (an essential trace element) plays a vital role in the human body by improving the activity of the seleno-enzyme and glutathione peroxidase and preventing free radicals from damaging cells and tissues.6 Laboratory studies have also provided evidence that Se treatment could reduce the incidence of various cancers such as hepatocarcinoma and lung, colon, and prostate cancer.7 A few Se-containing compounds have antimicrobial activities and have applications in pharmaceutical industries, for example, the use of selenium sulfide in fungicides and antidandruff shampoo manufacturing.8Furthermore, the high reactivity of Se metal nanoparticles towards a variety of chemicals enabled the synthesis of many other fundamental selenium functional metal chalcogenides such as ZnSe, CdSe, Ag2Se, SnSe, etc.4,9–11 One of the major downsides of the use of Se for biomedical applications is that at higher doses, Se is more toxic; however, Se NPs have a seven-fold lower acute toxicity than other biologically available forms such as sodium selenite and selenite12. Physicochemical methods are popular for the development of various Se NPs as they generate Se in large quantities with different forms in a short time. However, in both methods, the use of toxic chemicals is unavoidable, which basically impede their direct biomedical uses.13 Secondly, these techniques are complicated, expensive, and result in the production of harmful toxic waste as byproducts, which are dangerous not only to the ecosystem but also to humans. A biogenic approach for Se NP synthesis can be regularly achieved by the reduction of selenate/selenite in the presence of bacterial cells or their protein or plant extracts containing phenols, flavonoids, alcohols, aldehydes, etc.14 The microbial synthesis of Se NPs is a simple detoxification process, in which toxic selenate and selenite oxyions are reduced to non-toxic elemental Se and the reverse reaction is too slow to produce Se compounds.15 There are two modes used for reducing oxyions, i.e. anaerobic and aerobic. Large scale bio-manufacturing processes for the synthesis of Se NPs through anaerobic modes are very time-consuming and challenging. This is why researchers are interested in finding aerobic microorganism modes that can endure a high concentration of Se oxyions and reduce them to elemental Se within a NP size range.16
Se NPs are useful in various emerging applications such as antifungal agents, anti-cancer agents, orthopedic implants, treatment of malignant mesothelioma, etc.17,18 From an environmental perspective, Se NPs capture mercury from the gaseous phase and it precipitates on the NP surface as HgSe.19,20 This is why there is an increase in the demand for Se NPs in everyday life as well as in industrial areas, and to fulfil these demands, there is a vital need to increase their production yield, for which optimization of the process is very important step. A few contributions have been made in the literature previously regarding the effect of the cultural and physical conditions on the biosynthesis of Se NPs.16 The synthesis of Se NPs with nano-range sizes in high yields is still a challenging issue. In order to increase the yield and the shelf-life (stability) of Se NPs with minimum investment, it is essential to optimize the cultural conditions and various physical parameters such as pH, light intensity, temperature, etc. To our knowledge, there has been no prior report on the optimization of all these conditions (parameters) for the large scale synthesis of Se NPs. We report the optimization of different conditions including medium, temperature, pH, light intensity, concentration of selenium dioxide (SeO2), and time of reaction for the bio-synthesis of Se NPs via Pantoea agglomerans.
2. Material and methods
2.1 Isolation of the organisms
In the present study, about 28 soil samples were collected from various farm fields of the Hingoli district (Aundha and Basmatnagar) and from different parts of the Lonar lake in the Buldhana district in the Eastern part of Maharashtra, i.e. the middle part of India. Soil samples were collected from a depth of 1–15 inches from the surface and sieved through a 2 mm sieve. UV sterilized polythene bags were used for the collection of the soil samples so as to prevent any external contamination and stored at 4 °C for further studies. A 100 g soil sample was placed in a sterile beaker and 0.5 mM of SeO2 was added to it. A small quantity of water (equivalent to 60% of the water holding capacity) was added into it and incubated for 30 days enrichment. Tryptic soya agar21 supplemented with SeO2 (0.5 mM) was used for isolation. All the ingredients were weighed to the desired volume and made up to 250 mL in an Erlenmeyer flask and sterilized at 121 °C for 15 min according to the standard procedure.16 After sterilization of the media filter, sterilized SeO2 was added to medium when the temperature reached 40–45 °C. Aliquots of 0.5 mL of the serially diluted enrichment broth was inoculated under aseptic conditions into Petri-plates containing the selective medium and the plates were incubated at 37 °C for 24 to 48 h in an inverted position in the incubator for the development of colonies. These plates were maintained in replicates, SeO2 tolerance was checked by growing a pure culture under increasing concentrations of SeO2 (2–10 mM). Isolates showing the maximum tolerance to the SeO2 concentration were maintained on agar slants containing SeO2 and were used for further studies.2.2 Bacterial identification
The isolated bacteria were identified using morphological, biochemical, and molecular techniques. In the morphological identification, different staining techniques, such as Gram staining, endospore staining, motility tests, etc., were performed. Standard methods as described in Bergey's Manual of Systematic Bacteriology were used for the biochemical characterizations of the isolated bacterial strain.22 Molecular characterization was carried out through 16S rRNA gene analysis. The genomic DNA of the bacterial stain was isolated using the InstaGene™ Matrix Genomic DNA isolation kit followed by polymerase chain reaction (PCR, MJ Research Peltier Thermal Cycler) amplification with universal primers. The amplified product was purified and sequenced by the dideoxy chain termination method followed by capillary electrophoresis on an ABI 3730xl sequencer. The sequence obtained was BLAST searched and compared with sequences of other closely related species retrieved from the GenBank database http://www.ncbi.nlm.nih/gov/BLAST. The alignment was carried out using MEGA software version 4 (ref. 23) and a phylogenetic tree was constructed using the neighbour-joining algorithm. Confidence limits of the branching were assessing by bootstrap analysis.2.3 Culture and inoculums
The selected bacterial strain (hereon named E) was maintained on agar slants at 4 °C and sub-cultured every five days. The inoculum was cultured in 100 mL of tryptic soy broth (TSB) using 250 mL erlenmeyer flasks.242.4 Production
Inoculum 1% (v/v) was inoculated in 300 mL TSB supplemented with 9 mM SeO2 and incubated at 37 °C, 200 rpm for 48 h. Escherichia coli K-12 were used as a negative control. After 24 h, the bacterial cells were separated by centrifugation at 3000 rpm (Hermle centrifuge, Z36HK) at 4 °C for 10 min. The cell pellet was removed and the cell-free medium was again centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at 4 °C for 30 min. The supernatant was discarded and the pellet with the Se-containing NPs was re-suspended in deionised water. The suspension was washed twice with deionised water by repeating the two centrifugation steps.25
000 rpm at 4 °C for 30 min. The supernatant was discarded and the pellet with the Se-containing NPs was re-suspended in deionised water. The suspension was washed twice with deionised water by repeating the two centrifugation steps.25
2.5 Characterisation
The absorption spectrum of the red Se NPs suspended in an aqueous solution was recorded using a Jasco V-680 spectrophotometer with wavelengths in the range of 300–1000 nm using water as a blank. The morphologies and structures of the synthesized Se NPs were examined using X-ray diffraction (XRD) patterns (X-ray diffractometer Rigaku D/MAX 2500 V, Cu Kα, λ = 1.5418 Å) and plane-view of field-emission scanning electron microscopy (FE-SEM, Hitachi S-4200) digital photo-images, respectively.2.4 Optimization of the conditions
For the large scale and stable biosynthesis of Se NPs, it is necessary to optimize the physical and cultural conditions. Several experiments were carried out concerning the rate of synthesis and stability of the Se NPs. Parameters, such as medium, pH, temperature, light intensity, concentration of SeO2, time of reaction, etc., were standardized for the rapid and maximum yield of synthesized Se NPs. For each condition, there was a respective control. All experiments were performed in triplicate for the confirmation studies.3. Results and discussion
3.1 Cultural and molecular characterization of the isolate
Of all the collected soil samples, only seven soil samples from the field and from one site of the Lonar Lake yielded bacteria with the capacity to produce Se NPs under the conditions set. All the samples which showed positive growth on SeO2 containing media were from mustard or cabbage farm fields. This might be due to the fact that these crops could have the ability to accumulate high concentrations of Se around the rhizosphere of the plant.25 Nearly fifty-seven bacterial isolates were found to have the ability to form Se NPs as the colour of the reaction in respective solution was changed. E isolate was selected for further study as it was able to tolerate high concentrations of SeO2, i.e. up to 9 mM. According to morphological characterization, isolate E is a Gram-negative, facultative anaerobic, nonsporulating, rod shaped, flagellated bacterium. The temperature for growth of Se NPs ranged from 16–53 °C (optimum 40 °C), the pH was in the range of 4–11 (optimum pH ∼7.5), and the salt concentration (NaCl %) was up to 7.5%. Biochemical analysis was positive for catalase, inositol production, hydrolysis of gelatin, and Voges–Proskauer and negative for oxidase, citrate, methyl red, nitrate, and urea hydrolysis. Acid could be produced from carbohydrates namely dextrose, fructose, lactose, etc. The results of the 16S rRNA (1793 bp) gene sequence analysis of the strain suggested that it belonged to the genus Pantoea sp. (GenBank: KU500622). In the phylogenetic tree, the strain E formed a coherent branch with Pantoea agglomerans. The isolate E also showed 99% sequence similarity to Pantoea agglomerans. Therefore, on the basis of the above results, strain E was characterized as Pantoea agglomerans.3.2 Structure and morphology studies
The change in appearance of the bacterial culture from pale yellow to red was due to excitation of surface plasmons after treatment with SeO2, which indicates the formation of Se NPs (Fig. 1a inset) as in general SeO2 in solvent is transparent and colorless. As-synthesized Se NPs exhibited a strong absorption in the visible range due to the local surface plasmon resonance effect.13 The UV-Vis spectrum was recorded and is shown in Fig. 1a wherein an absorbance peak at ∼593 nm, caused by Se NPs, was observed. The presence of a single peak corroborated the successful synthesis of Se NPs. A very close relationship between the UV-Vis absorbance and the size and shape of Se NPs could be well-established. With increasing particle size, the optical absorption spectrum of the metal NPs was dominated by surface plasmon resonances shifting towards longer wavelengths (red-shift).27 A small blue-shift or red-shift in the wavelength of the absorbance peak might relate to the synthesis of Se NPs in different shapes and sizes. The XRD analysis showed that the Se NPs were amorphous in nature (Fig. 1b) as there was neither a hump nor sharp reflection peaks for different 2θ angles. Energy-dispersive X-ray spectroscopy (EDX) analysis gives the qualitative as well as quantitative status of elements present in the product. Fig. 1c shows the elemental profile of the synthesized Se NPs using bacterial E. The analysis revealed the highest proportion of Se in NPs without any trace of other impurity elements. | ||
| Fig. 1 (a) Absorption spectrum of Se NPs and colonies of Pantoea agglomerans strain E, (b) XRD spectrum, and (c) EDX mapping presenting the presence of Se. | ||
3.3 The effect of the synthesis medium
The bacterial synthesis of Se NPs is an enzyme catalyzed reaction; reductase plays an important role in biosynthesis.15,16 Bacterial cells react differently to different culture media and their components by secreting different metabolites and different types of proteins. For the maximum production of Se NPs by corresponding bacteria, the bacteria should secrete specific enzymes or metabolites in large quantities, which would otherwise be responsible for the reduction of Se oxyions. To evaluate the effect of different media on enzyme secretion and the synthesis of Se NPs, we grew bacterial cells on the previously mentioned six different media.16,21,28–30 In the present study, microbial growth and reduction of SeO2 (red appearance) were observed in Tryptic soya followed by DeMan–Rogosa–Sharpe (MRS) and then Luria–Bertani agar (LB agar). The maximum growth was found in Tryptic soya medium, which might promote the extracellular reductase secretion by enhancing the synthesis of Se NPs wherein the bacterial growth without SeO2 reduction was observed in R2A, nutrient agar and liquid modified marine medium. A surface study of the Se NPs synthesized by the selected bacterial strain in Tryptic soya, MRS and LB agar showed that the Se NPs synthesized in TSB possessed smaller particle sizes (80–85 nm), while larger particle sizes were confirmed from MRS, i.e. 100–300 nm, and LB broth, i.e. 100–200 nm (see Fig. 2a–c for more details). This might be due to capping of Se NPs by different extra-cellularly secreted proteins and also could have multiple effects on the dispersion, including potential screening of the surface charges, which otherwise would help in maintaining the repulsion between the particles, or bridging type interactions.31 | ||
| Fig. 2 The effect of growth media on the synthesis of Se NPs: (a) Tryptic soya broth, (b) DeMan–Rogosa–Sharpe, and (c) Luria–Bertani broth. | ||
3.4 The effect of pH
For the stable synthesis of Se NPs, pH plays an important role. Alkaline conditions (pH ≈ 8–9) showed the maximum yield for the synthesis of NPs, while acidic culture (pH ≈ 4–5) confirmed aggregation type behaviour. At pH ∼7, a stable synthesis of Se NPs but with considerably lower yield was observed and even after 45 days, no aggregates were seen. The alkaline pH revealed the maximum yield for the synthesis of Se NPs but the NPs were less stable than those obtained from neutral pH, in which aggregates were observed after 38 days. This might be because alkaline pH could exert a positive effect on the expression level of enzymes. Previously, it has been noted that alkaline conditions can increase the production of 243 enzymes, which are not only responsible for the synthesis of Se NPs but other functions also.32 Here, –OH is very much required for the reduction of metal ions, which is why there was an enhancement of the synthesis towards alkaline pH.31 A high negative charge was found on Se NPs synthesized at pH ∼7 (−44.02 mV) and ∼9 (−42.56 mV), confirmed after zeta potential measurements. If there is a highly negative or positive zeta potential on the Se NPs, then they tend to repel each other and have a tendency to minute aggregation in the solution. A highly negative charge on the Se NPs at pH ∼7 and ∼9 was probably as a result of the high stability and low tendency to form aggregates. These particles might not transform to the black amorphous form when kept for a prolonged period of time; therefore, a neutral pH ensures a long shelf life and greater stability and an alkaline pH is responsible for the maximum yield in the synthesis of Se NPs.3.5 The effect of temperature on extracellular protein secretion and the rate of Se NP synthesis
The rate of any chemical or biological reaction is affected by an important factor, temperature. The green synthesis of Se NPs is an enzyme mediated process and enzymes are temperature sensitive.33 In order to study the effect of temperature on extracellular protein secretion and the synthesis of Se NPs, bacterial cells were exposed to diverse temperatures from 0 to 100 °C with an interval of 10 °C. The maximum bacterial growth was observed at 40–50 °C and maximum extracellular protein synthesis was observed at 60 °C, which might be due to the heat shock (Fig. 3). A gradual increase in surface plasmon absorbance was observed with increasing temperature up to 50 °C, after which it decreased (Fig. 4). A special type of reductase, responsible for Se NP synthesis, showed an optimum activity at 50 °C. The increase in the absorbance indicated the increase in the number of Se NPs or an increase in the individual Se NP size. Although the maximum yield of synthesized protein was observed in 60 °C, the maximum yield in the Se NPs synthesis was confirmed at 50 °C. This might be due to the fact that the bacterial cell could synthesize some other proteins in large quantities, not responsible for Se NP synthesis (Fig. 3 and 4). Surface analysis of the Se NPs synthesized at 50 °C revealed an average particle size of 60 nm and surprisingly there was no agglomeration up to 43 days. On the other hand, Se NPs developed at 30 °C confirmed an average particle size of 100 nm and those at 40 °C demonstrated an average particle size of 100 nm and were irregular and less chemically and environmentally stable. A higher average particle size was recorded at 60 °C, i.e. 110–150 nm (Fig. 5). It was noted that bacterial culture maintained at 70–100 °C was unable to produce any bacterial growth as there was no colour change, indicating the unsuitability of this temperature range for the production of Se NPs. | ||
| Fig. 5 The effect of temperature on Se NP synthesis by Pantoea agglomerans at (a) 30, (b) 40, (c) 50, and (d) 60 °C. | ||
3.6 The effect of light intensity
Photochemical routes were frequently used for the synthesis of metal NPs during the 18th century. Currently, a variety of photoinduced synthetic methods for metal nanostructures are being developed to achieve well-defined monometallic and bimetallic NPs in addition to their composite materials.34 In order to study the effect of light intensity, bacterial cells were exposed to various light intensities, as discussed earlier. In presence of sunlight, a rapid reduction (12 h) in the concentration of Se oxyions took place, which produced a sharp peak at ∼594 nm. The rapid extracellular synthesis in sunlight might be due to the free electrons of aromatic compounds generated after the photosensitization of aromatic compounds in medium, which could be utilized by Se oxyions for reduced NPs. Under 189.9 and 141.9 Lux light intensities, a symmetric graph with a narrow size-distribution was confirmed. However, more time was required than usual for completing the reduction of the Se oxyions, i.e. 48 h. At 14.5 and 94.1 Lux intensities, even after 72 h, there was no evidence for Se NPs synthesis. The absorbance increased with increasing light intensity. Interestingly, synthesis of Se NPs was also found to occur in the dark but the process was extremely slow, i.e. after 72 h there was slight colour change.3.7 The effect of the Se oxyion concentration
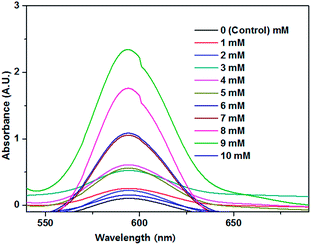
The synthesis of Se NPs is an enzymatic process; substrate concentration plays an important role in the achievement of the maximum yield of product. To obtain a maximum yield of Se NPs during the synthesis, bacterial cells were exposed to increasing concentrations of Se oxyions. The highest yields for the syntheses of Se NPs were observed at a Se oxyion concentration of 9 mM. A higher concentration than 9 mM did not reveal any bacterial growth and no visible colour change was observed, which could be because Se oxyions become toxic to bacterial cells at higher concentrations, obviously affecting the growth of Se NPs during synthesis (Fig. 6).3.8 The effect of reaction time
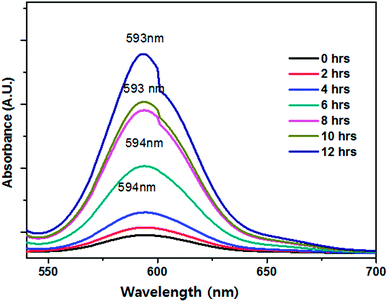
To study the influence of the reaction time, we measured the UV-Vis spectra of the resulting samples at regular time intervals from 2 to 12 h. Initially, the colour of the filtrate was pale-yellow, and as time passed, the colour changed to red followed by dark-red with an increase in the absorbance, indicating the continuous growth of Se NPs in the filtrate (Fig. 7).The Se NPs synthesized by this optimised method were utilized for mitigating the side effects of enrofloxacin, which is the most commonly used antibiotic in broiler chicken. Enrofloxacin has been reported previously for the generation of immunological and antioxidant studies in poultry. There was a decrease in the cellular (phytohemagglutinin (PHA) test, ratio of heterophils and lymphocytes (H/L)) and humoral immune (HI, immunoglobulin G (IgG) and immunoglobulin M (IgM)) responses. Enrofloxacin also significantly affected both the enzymatic (superoxide dismutase (SOD), catalase (CAT), and phospholipid hydroperoxide glutathione peroxidase (GSH-Px)) and nonenzymatic (glutathione (GSH)) antioxidants and also the lipid peroxidation (malondialdehyde), causing oxidative stress in broiler chickens. Scheduled oral administration of biogenic Se NPs (0.3 to 0.6 mg per kg of feed) helped to reduce this immunological and oxidative stress.35
4. Conclusions
Exploitation of a natural source for the synthesis of Se NPs can overcome the problem of energy consumption as well as the rate of synthesis. Microorganisms are a prospective source for the synthesis of Se NPs, which have applications in non-biological fields, such as in photocatalysis, rectifiers, sensors, and solar cells, and biological fields such as anticancer, antimicrobial, and antiprotozoal agents, as dietary supplementation. Cultural (culture medium) and physical conditions (pH, temperature, reaction time, oxyion concentration, and light intensity) have an influence on the yield of Se NPs. Rapid, stable, and small-sized Se NPs synthesis was achieved from tryptic soya medium maintained at pH ∼9, temperature ∼50 °C with 9 mM Se oxyion concentration. The optimization of the parameters would lead to the rapid and large scale production of Se NPs, useful at the industrial level, which may have further applications in medicine and health care products.Acknowledgements
The authors acknowledge the King Saud University, Deanship of Scientific Research, College of Science Research Center for the support. This research was supported by Global Frontier Program, through the Global Frontier Hybrid Interface Materials (GFHIM) of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2013M3A6B1078874).Notes and references
- D. Huber, Small, 2005, 1, 482 CrossRef CAS PubMed.
- S. Walsh, J. M. Balbus, R. Denison and K. Florini, J. Cleaner Prod., 2008, 16, 1018 CrossRef.
- X. Qu, P. J. J. Alvarez and Q. Li, Water Res., 2013, 47, 3931 CrossRef CAS PubMed.
- C. An and S. Wang, Mater. Chem. Phys., 2007, 101, 357 CrossRef CAS.
- C. H. An, K. B. Tang, X. M. Liu and Y. T. Qian, Eur. J. Inorg. Chem., 2003, 2, 3250 CrossRef.
- S. Y. Zhang, J. Zhang, H. Y. Wang and H. Y. Chen, Mater. Lett., 2004, 58, 2590 CrossRef CAS.
- Q. Li, T. Chen, F. Yang, J. Liu and W. Zheng, Mater. Lett., 2010, 64, 614 CrossRef CAS.
- M. Lenz and P. N. L. Lens, Sci. Total Environ., 2009, 407, 3620 CrossRef CAS PubMed.
- Y. T. Chen, W. Zhang, Y. Q. Fan, X. Q. Xu and Z. X. Zhang, Mater. Chem. Phys., 2006, 98, 191 CrossRef CAS.
- C. Dwivedi, C. P. Shah, K. Singh, M. Kumar and P. N. Bajaj, J. Nanotechnol., 2011, 12, 1–6 CrossRef.
- Y. Ren, M. Niu, W. Gu and Y. Fang, Mater. Lett., 2012, 82, 148 CrossRef CAS.
- J. S. Zhang, H. L. Wang, X. X. Yan and L. D. Zhang, Life Sci., 2005, 76, 1099 CrossRef CAS PubMed.
- S. Shirsat, A. Kadam, M. Naushad and R. S. Mane, RSC Adv., 2015, 5, 92799 RSC.
- A. Husen and S. Siddiqi, J. Nanobiotechnol., 2014, 12, 28 CrossRef PubMed.
- H. DeMoll-Decker and J. M. Macy, Arch. Microbiol., 1993, 160, 241 CAS.
- S. Dhanjal and S. Cameotra, Microb. Cell Fact., 2010, 9, 1 CrossRef PubMed.
- T. J. Webster, IEEE 33rd Annual Northeast Bioengineering Conference, NEBC, 2007, 07, 241 Search PubMed.
- A. R. Shahverdi, A. Fakhimi, G. Mosavat, P. Jafari-Fesharaki, S. Rezaie and S. M. Rezayat, World Appl. Sci. J., 2010, 10, 918 CAS.
- N. C. Johnson, S. Manchester, L. Sarin, Y. Gao, I. Kulaots and R. H. Hurt, Environ. Sci. Technol., 2008, 42, 5772 CrossRef CAS PubMed.
- J. W. Fellowes, R. A. D. Pattrick, D. I. Green, A. Dent, J. R. Lloyd and C. I. Pearce, J. Hazard. Mater., 2011, 189, 660 CrossRef CAS PubMed.
- M. Losi and W. T. Frankenberger, Appl. Environ. Microbiol., 1997, 63, 3079 CAS.
- D. H. Bergey, E. R. Buchanan and N. E. Gibbons, Bergey's Manual of Determinative Bacteriology, Williams & Wilkins, Baltimore, 18th edn, 1974 Search PubMed.
- K. Tamura, J. Dudley, M. Nei and S. Kumar, Mol. Biol. Evol., 2007, 24, 1596 CrossRef CAS PubMed.
- S. K. Torres, V. L. Campos, C. G. Leon, S. M. Rodriguez-Llamazares, S. M. Rojas, M. Gonzalez, C. Smith and M. A. Mondaca, J. Nanopart. Res., 2012, 14, 1236 CrossRef.
- T. Wang, L. Yang, B. Zhang and J. Liu, Colloids Surf., B, 2010, 80, 94 CrossRef CAS PubMed.
- O. A. Beath, C. S. Gilbert and H. F. Eppson, Am. J. Bot., 1939, 26, 257 CrossRef CAS.
- G. A. Shafeev, E. Freysz and B. Verduraz, Appl. Phys. A, 2004, 78, 307 CrossRef CAS.
- W. J. Hunter and D. K. Manter, Curr. Microbiol., 2009, 58, 493 CrossRef CAS PubMed.
- P. J. Fesharaki, P. Nazari, M. Shakibaie, S. Rezaie, M. Banoee, M. Abdollahi and A. R. Shahverdi, Braz. J. Microbiol., 2010, 41, 461 CrossRef CAS PubMed.
- L. Lortie, W. D. Gould, S. Rajan, R. G. L. Meeready and K. J. Cheng, Appl. Environ. Microbiol., 1992, 58, 4042 CAS.
- I. Montes-Burgos, I. Salvati, I. Lynch and K. Dawson, Proceedings of the European Science Foundation Research Conference on Probing Interactions between Nanoparticles/Biomaterials and Biological Systems, SantFeliudeGuixols, Spain, November 2007 Search PubMed.
- R. Sanghi and P. Verma, Chem. Eng. J., 2009, 155, 886 CrossRef CAS.
- J. Dobias, E. I. Suvorova and R. Bernier-Latmani, Nanotechnology, 2011, 22, 195605 CrossRef CAS PubMed.
- T. Triantis, A. Troupis, E. Gkika, G. Alexakos, N. Boukos, E. Papaconstantinou and A. Hiskia, Catal. Today, 2009, 144, 2 CrossRef CAS.
- S. Shirsat, A. Kadam, R. S. Mane, V. V. Jadhav, M. K. Zate, M. Naushad and K. H. Kim, Dalton Trans., 2016 10.1039/c6dt00120c.
| This journal is © The Royal Society of Chemistry 2016 |