An urchin-like Ni3ZnC0.7–carbon nanotube-porous carbon composite derived from metal–organic gel as a cathode material for rechargeable Li–O2 batteries†
Abstract
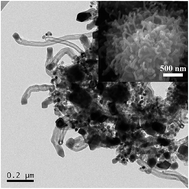
An urchin-like Ni3ZnC0.7–carbon nanotube-porous carbon composite was synthesized, for the first time, by one-step direct thermolysis of a metal–organic gel in a conventional horizontal tube furnace without using any additional carrier gas or catalyst. The urchin-like particles of the obtained composite with a particle size of ∼2 μm exhibit a sphere-like core, and CNTs grow outward from the core. And the Ni3ZnC0.7 nano-particles are embedded in the composite, including at the end of the nanotubes. When used as the cathode material of Li–O2 batteries, the composite exhibits excellent electrochemical performances, delivering a good cycle performance and having a discharge capacity of ∼7390 mA h g−1carbon+catalyst at 0.1 mA cm−2.

 Please wait while we load your content...
Please wait while we load your content...