A facile and efficient method for the synthesis of 1,2,4-trisubstituted imidazoles with enamides and benzylamines†
Jinhui Cao,
Xiaoqiang Zhou ,
Haojie Ma,
Chong Shi and
Guosheng Huang*
,
Haojie Ma,
Chong Shi and
Guosheng Huang*
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, Department of Chemistry, Lanzhou University, Lanzhou, 730000, China. E-mail: hgs@lzu.edu.cn; Fax: +86-931-8912582
First published on 8th June 2016
Abstract
A novel and practical method for the construction of 1,2,4-trisubstituted imidazoles with enamides and benzylamines catalysed by CuBr and I2 has been developed. This sustainable, simple and environmentally-friendly procedure tolerates various functional groups and affords a series of trisubstitued imidazoles.
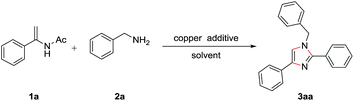
Imidazoles are one of the most important N-containing heterocyclic compounds, which not only have been found in a large number of natural products,1 but also have been widely applied in functional materials.2 Especially, they also have good pharmacological activities, such as antitumor,3 antifungal,4 antiplasmodium,5 antibacterial,6 and anti-inflammatory activities.7 Giving the credit to their long lasting significance and broad applications, a number of methods have been developed for the synthesis of imidazole derivatives. Among them, the most fashionable way to trisubstituted imidazoles is the condensation of diketones, aldehydes with amines or ammonias.8 A number of catalysts for this process have been reported, such as acetic acid,9 silica sulphuric acid,10 NiCl2·H2O/Al2O3,11 ZrCl4,12 ionic liquids,13 and CAN.14 Recently, transition-metal-catalyzed cross-coupling reactions have been considered as an effective and powerful strategy to construct C–N bond directly.15 Copper was naturally considered to be one of the most efficient, inexpensive and persistent ones for C–N bond formation.16 Lately, the copper-catalyzed synthesis of 1,2,4-trisubstituted imidazoles from amidines with terminal alkynes17 and ketones with benzylamines18 has been developed. Despite great advances have been made for the construction of trisubstituted imidazoles relying on amines derivatives, many of these procedures suffer from one or more disadvantages such as complex starting material, harsh reaction conditions and amounts of expensive catalysts. Therefore the development of efficient and general access to 1,2,4-trisubstituted imidazoles is of great challenge. Enamides are naturally considered to be one of the most excellent and significant synthons which have been illuminated by copper catalyzed reaction for N-heterocycles synthesis.19 Very recently, our group have reported several novel methods for the conversion of enamides to N-heterocycles.20 As a continued work, herein, we report a novel and facial process to obtain substituted imidazoles from enamides and benzylamines.
To explore the optimal reaction conditions, we initiate the reaction of enamides 1a (0.2 mmol) with benzylamines 2a (0.6 mmol) in the presence of CuBr2 (0.1 eq.) and LiBr (1.5 eq.) in Toluene at 60 °C for 8 h. As expected, the desired product trisubstitued imidazole 3aa was obtained in 39% yield (Table 1, entry 1). Then, the effect of various Cu-salts (e.g. CuCl2, Cu(OAc)2, CuI, CuBr, CuCl) on the yield of the target product was investigated to find that CuBr can successfully improve the yield to 56% (Table 1, entries 2–6). Subsequently, different additive were screened, and I2 displayed better result for the constructing of the relevant imidazole than others such as KBr, NIS and NBS. Then, the desired product was detected when DMF, PhCl, CH3CN, 1,4-dioxane, DCE were used as the solvents, respectively. The results illustrated 1,4-dioxane was better solvents than others in this transformation (Table 1, entries 12–16). Gratifyingly, the highest yield was given after adding 0.05 eq. I2 in 1,4-diaxone at 60 °C (Table 1, entry 15). The manifestation of the reaction did not give a superior result with the change in temperature (40 °C, 80 °C, 100 °C) or reaction time (12 h and 24 h). Finally, the optimized reaction conditions were determined as follows: 1,4-diaxone as solvent, 10 mol% CuBr as catalyst, 5 mol% I2 as additive, reaction temperature at 60 °C.
| Entry | Catalyst | Additive | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Conditions: 1a (0.2 mmol), 2a (0.6 mmol), catalyst (0.1 equiv.), additive (0.05 equiv.), solvent (2 mL), under 60 °C monitored by TLC. DMF = dimethylformamide, DCE = 1,2-dichloroethane, PhCl = chlorobenzene, CH3CN = acetonitrile.b Isolated yields.c nd = not detected. | ||||
| 1 | CuBr2 | LiBr | Toluene | 39 |
| 2 | CuCl2 | LiBr | Toluene | ndc |
| 3 | Cu(OAc)2 | LiBr | Toluene | nd |
| 4 | CuI | LiBr | Toluene | 34 |
| 5 | CuBr | LiBr | Toluene | 56 |
| 6 | CuCl | LiBr | Toluene | nd |
| 7 | CuBr | KBr | Toluene | nd |
| 8 | CuBr | NBS | Toluene | nd |
| 9 | CuBr | I2 | Toluene | 65 |
| 10 | CuBr | NIS | Toluene | nd |
| 11 | CuBr | — | Toluene | nd |
| 12 | CuBr | I2 | DMF | nd |
| 13 | CuBr | I2 | PhCl | 25 |
| 14 | CuBr | I2 | CH3CN | nd |
| 15 | CuBr | I2 | 1,4-Diaxone | 78 |
| 16 | CuBr | I2 | DCE | nd |
With the optimized reaction conditions in hand, a series of enamides with electrondonating or withdrawing groups were investigated (Scheme 1). Generally, the reactions afforded the desired substituted imidazoles in moderate yields and displayed high functional groups tolerance including methyl, methoxyl, phenyl, fluoro, chloro, bromo, trifuoromethyl (3ba–3la). The yields remained relatively stable with the nature of the different groups in aromatic ring of enamides, and regioselectivity favored the least sterically hindered position. For example, enamides with groups of 4-methyl and 3-methyl, gave the corresponding products (3ba, 3ca) in 76% and 72% yields, respectively. However, when using 2-methyl enamide as reactant, corresponding product was not detected from TLC measurement (3da). Substituted enamides with 4-phenyl and 2-naphthyl on the benzene ring could provide the corresponding products (3la, 3na) in 73% and 61% yields, respectively. When using 1-naphthyl enamide as reactant, the corresponding product (3oa) was only obtained in 32% yields. Furthermore, the reaction with halogenated enamides gave the corresponding products (3ha–3ja, 3ma, 3pa) in good yields. In addition, enamide with strong electron-withdrawing group such as 4-CF3 could transformed smoothly with a moderate yield (3ka). To our disappointment, the use of internal alkene such as N-(3,4-dihydronaphthalen-1-yl)acetamide (1q) and N-(1H-inden-3-yl)acetamide (1r) couldn't occur in standard conditions.
The process was further expanded to a range of substituted benzylamines (Scheme 2). The results demonstrated that substituents at different positions of benzene ring (para, meta, and ortho position) could affect the efficiency obviously. Either electrondonating or withdrawing groups at para-position of benzylamine all can transform successfully and provided the desired imidazoles in moderate to good yields (3ab, 3ac, 3af–3ah). However, the group at meta, and ortho position of benzylamine can give the desired product in an apparent reduction (3ad, 3ae, 3ai–3ak). Substituent –OMe at ortho-position on the aroma ring gave an obviously reduced yield (3ae, 52%), compared to the one at para-position (3ac, 73%). The desired product was only obtained in 29% when –OMe was at meta-position (3ad), which indicated the influence of steric effect on the construction of the targets. Moreover, the process was also extended to 1-naphthyl benzylamine and generated the desired 3al in 60% yield. Meanwhile, the tryptamine was also employed for this reaction, to our disappointment, tryptamine did not suit for this conversion. Further investigation revealed that aliphatic amines such as n-butylamine (2n) and 1-aminodecane (2o) are not suitable for the reaction.
In order to obtain further insights into this reaction, several control experiments were investigated (Scheme 3). We first conducted the reaction of 1a and 2a in the presence of 2.0 equiv. of TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) under optimized conditions, 72% 3aa was obtained. This result showed that the reaction might not undergo the radical pathway. Then the substrate 1a was employed to reaction with imine 4 under optimized conditions, the final product was not detected. The result revealed that the compound 4 should not be the intermediate in this transformation.
According to the aforementioned results and previous reports,18,21 the possible mechanism for this transformation is proposed as illustrated in Scheme 4. Initially, the intermediate A was produced by 1a and 2a under copper/oxygen system. Subsequently, A attacked the imine B, which was generated by oxidation of 2a, to provide the intermediate C. Then intramolecular amidination of isomerized D afforded intermediate E. Subsequently, intermediate G was formed by the oxidation and hydrolysis process of E. Finally, the aromatization of dihydroimidazole G by copper/iodine oxidation process gave the desired 3aa.
Conclusions
In summary, we have described a novel and efficient strategy for the synthesis of substituted imidazoles in one step using easily available starting materials catalyzed by available CuBr/I2. The reaction is convenient, and environmentally friendly with good yields. Moreover, this strategy for 1,2,4-trisubstituted imidazoles might be an efficiently method in organic, material, and medicinal chemistry.Notes and references
- (a) D. L. Luca, Curr. Med. Chem., 2006, 13, 1 Search PubMed; (b) J. Zhong, Nat. Prod. Rep., 2009, 26, 382 RSC; (c) F. Xiong, X. Chen and F. Chen, Tetrahedron: Asymmetry, 2010, 21, 665 CrossRef CAS; (d) M. Roue, I. DomartCoulon, A. Ereskovsky, C. Djediat, T. Perez and M. L. Bourguet-Kondracki, J. Nat. Prod., 2010, 73, 1277 CrossRef CAS PubMed.
- (a) Y. Maeda, T. Nishimura and S. Uemura, Bull. Chem. Soc. Jpn., 2003, 76, 2399 CrossRef CAS; (b) J. A. Asensio and P. G. Romero, Fuel Cells, 2005, 5, 336 CrossRef CAS; (c) N. Singh and D. O. Jang, Org. Lett., 2007, 9, 1991 CrossRef CAS PubMed; (d) N. Nagarajan, G. Velmurugan, A. Prakash, N. Shakti, M. Katiyar, P. Venuvanalingam and R. Renganathan, Chem.–Asian J., 2014, 9, 294 CrossRef CAS PubMed; (e) J. E. Kwon, S. Park and S. Y. Park, J. Am. Chem. Soc., 2013, 135, 11239 CrossRef CAS PubMed; (f) C. Lee, Y. Yuan, J. Chen, F. Lu, Q. Tong, Q. Yang and H. Mo, Chem. Mater., 2013, 25, 4957 CrossRef; (g) A. Jeżewski, T. Hammann, P. J. Cywiński and D. T. Gryko, J. Phys. Chem. B, 2015, 119, 2507 CrossRef PubMed.
- (a) M. Fukui, M. Inaba, S. Tsukagoshi and Y. Sakurai, Cancer Res., 1982, 42, 1098 CAS; (b) G. J. Atwell, J. Fan, K. Tan and W. A. Denny, J. Med. Chem., 1998, 41, 4744 CrossRef CAS PubMed; (c) S. Y. Al-Raqa, A. M. S. ElSharief, S. M. E. Khalil and A. M. Al-Amri, Heteroat. Chem., 2006, 7, 643 Search PubMed.
- (a) D. J. Wolff, G. A. Datto and R. A. Samatovicz, J. Biol. Chem., 1993, 268, 9430 CAS; (b) N. Sennequier, D. Wolan and D. J. Stuehr, J. Biol. Chem., 1999, 274, 930 CrossRef CAS PubMed; (c) H. Koga, Y. Nanjoh, K. Makimura and R. Tsuboi, Med. Mycol., 2009, 47, 640 CrossRef CAS PubMed; (d) V. Zoete, O. Michielin, U. F. Rohrig, S. R. Majjigapu, M. Chambon, S. Bron and L. Pilotte, Eur. J. Med. Chem., 2014, 84, 284 CrossRef PubMed.
- J. Z. Vlahakis, C. Lazar, I. E. Crandall and W. A. Szarek, Bioorg. Med. Chem., 2010, 18, 6184 CrossRef CAS PubMed.
- (a) A. Vijesh, A. M. Isloor, S. Telkar, S. Peethambar, S. Rai and N. Isloor, Eur. J. Med. Chem., 2011, 46, 3531 CrossRef CAS PubMed; (b) W. R. Roush, J. Y. Choi, M. S. Plummer, J. Starr, C. R. Desbonnet, H. Soutter and J. Chang, J. Med. Chem., 2012, 55, 852 CrossRef PubMed; (c) L. Yurttas, M. Duran, S. . Demirayak, H. K. Gençer and Y. Tunalı, Bioorg. Med. Chem. Lett., 2013, 23, 6764 CrossRef CAS PubMed.
- (a) J. C. Lee, J. T. Laydon and P. C. Mcdonnell, Nature, 1994, 372, 739 CrossRef CAS PubMed; (b) J. L. Adams, J. C. Boehm, T. F. Gallagher, S. Kassis, E. F. Webb, R. Hall, M. Sorenson, R. Garigipati, D. E. Griswoldc and J. C. Lee, Bioorg. Med. Chem. Lett., 2001, 11, 2867 CrossRef CAS PubMed.
- (a) F. R. Japp and H. Robinson, J. Chem. Soc., Trans., 1882, 41, 323 RSC; (b) S. E. Wolkenberg, D. D. Wisnoski, W. H. Leister, Y. Wang, Z. Zhao and C. W. Lindsley, Org. Lett., 2004, 6, 1453 CrossRef CAS PubMed; (c) Y. Zhong, J. Lee, R. A. Reamer and D. Askin, Org. Lett., 2004, 6, 929 CrossRef CAS PubMed; (d) S. Zaman, K. Mitsuru and A. D. Abell, Org. Lett., 2005, 7, 609 CrossRef CAS PubMed; (e) A. R. Karimi, Z. Alimohammadi, J. Azizian, A. A. Mohammadi and M. R. Mohammadizadeh, Catal. Commun., 2006, 7, 728 CrossRef CAS; (f) M. M. Heravi, F. Derikvand and M. Haghighi, Monatsh. Chem., 2008, 139, 31 CrossRef CAS; (g) B. Sadeghi, B. B. F. Mirjalili and M. M. Hashemi, Tetrahedron Lett., 2008, 49, 2575 CrossRef CAS; (h) S. Samai, G. C. Nandi, P. Singh and M. S. Singh, Tetrahedron, 2009, 65, 10155 CrossRef CAS; (i) C. Mukhopadhyay, P. K. Tapaswi and M. G. B. Drew, Tetrahedron Lett., 2010, 51, 3944 CrossRef CAS; (j) K. Sivakumar, A. Kathirvel and A. Lalitha, Tetrahedron Lett., 2010, 51, 3016 CrossRef; (k) C. Y. Chen, W. P. Hu, P. C. Yan, G. C. Senadi and J. J. Wang, Org. Lett., 2013, 15, 6116 CrossRef CAS PubMed; (l) J. Li and L. Neuville, Org. Lett., 2013, 15, 1752 CrossRef CAS PubMed; (m) H. Huang, X. Ji, W. Wu and H. Jiang, Adv. Synth. Catal., 2013, 355, 170 CrossRef CAS; (n) S. Tong, Q. Wang, M. Wang and J. Zhu, Angew. Chem., Int. Ed., 2015, 54, 1293 CrossRef CAS PubMed; (o) L. Long, Y. Shao, L. Zhang and X. Zhou, Chem.–Eur. J., 2014, 20, 8551 CrossRef PubMed; (p) X. Guo, J. Shao, H. Liu, B. Chen, W. Chen and Y. Yu, RSC Adv., 2015, 5, 51559 RSC.
- (a) J. Wang, R. Mason, D. V. Derveer, K. Feng and X. R. Bu, J. Org. Chem., 2003, 68, 5415 CrossRef CAS PubMed; (b) S. Sarshar, D. Siev and M. M. Mjalli, Tetrahedron Lett., 1996, 37, 835 CrossRef CAS; (c) T. F. Gallagher, G. L. Seibel, S. Kassis, J. T. Laydon, M. J. Blumenthal, J. C. Lee, D. Lee, J. C. Boehm, S. M. Fier-Thompson, J. W. Abt, M. E. Soreson, J. M. Smietana, R. F. Hall, R. S. Garigipati, P. E. Bender, K. F. Erhard, A. J. Krog, G. A. Hofmann, P. L. Sheldrake, P. C. McDonnell, S. Kumar, P. R. Young and J. L. Adams, Bioorg. Med. Chem., 1997, 5, 49 CrossRef CAS PubMed.
- A. Shaabani and A. Rahmati, J. Mol. Catal. A: Chem., 2006, 249, 246 CrossRef CAS.
- M. M. Heravi, K. Bakhtiari, H. A. Oskooie and S. Taheri, J. Mol. Catal. A: Chem., 2007, 263, 279 CrossRef CAS.
- G. V. M. Sharma, Y. Jyothi and P. S. Lakshmi, Synth. Commun., 2006, 36, 2991 CrossRef CAS.
- S. A. Siddiqui, U. C. Narkhede, S. S. Palimkar, T. Daniel, R. J. Lahoti and K. V. Srinivasan, Tetrahedron, 2005, 61, 3539 CrossRef CAS.
- J. N. Sangshetti, N. D. Kokare, S. A. Kotharkara and D. B. Shinde, J. Chem. Sci., 2008, 5, 463–467 CrossRef.
- (a) J. Wencel-Delord, T. Droge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740 RSC; (b) T. A. Ramirez, B. Zhao and Y. Shi, Chem. Soc. Rev., 2012, 41, 931 RSC; (c) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC; (d) Z. Shi, C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3381 RSC.
- (a) A. E. Wendlandt, A. M. Suess and S. S. Stahl, Angew. Chem., Int. Ed., 2011, 50, 11062 CrossRef CAS PubMed; (b) C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3464 RSC; (c) S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234 CrossRef CAS PubMed; (d) X. Guo, D. Gu, Z. Wu and W. Zhang, Chem. Rev., 2015, 115, 1622 CrossRef CAS PubMed.
- J. Li and L. Neuville, Org. Lett., 2013, 15, 1752 CrossRef CAS PubMed.
- Z. Cai, S. Wang and S. Ji, Org. Lett., 2012, 23, 6068 CrossRef PubMed.
- (a) Z. Guan, Z. Zhang, Z. Ren, Y. Wang and X. Zhang, J. Org. Chem., 2011, 76, 339 CrossRef CAS PubMed; (b) S. Rakshit, F. W. Patureau and F. Glorius, J. Am. Chem. Soc., 2010, 132, 9585 CrossRef CAS PubMed; (c) D. Stuart, P. Alsabeh, M. Kuhn and K. Fagnou, J. Am. Chem. Soc., 2010, 132, 18326 CrossRef CAS PubMed; (d) M. Zhao, Z. Ren, Y. Wang and Z. Guan, Chem. Commun., 2012, 48, 8105 RSC; (e) M. Chen, Z. Ren, Y. Wang and Z. Guan, Angew. Chem., Int. Ed., 2013, 52, 14196 CrossRef CAS PubMed; (f) M. Zhao, Z. Ren, Y. Wang and Z. Guan, Chem.–Eur. J., 2014, 20, 1839 CrossRef CAS PubMed; (g) M. Zhao, Z. Ren, Y. Wang and Z. Guan, Org. Lett., 2014, 16, 608 CrossRef CAS PubMed; (h) B. Li, N. Wang, Y. Liang, S. Xu and B. Wang, Org. Lett., 2013, 15, 136 CrossRef CAS PubMed.
- (a) X. Zhou, H. Yan, C. Ma, Y. He, Y. Li, J. Cao, R. Yan and G. Huang, J. Org. Chem., 2016, 81, 25 CrossRef CAS PubMed; (b) Y. Li, X. Zhou, Z. Wu, J. Cao, C. Ma, Y. He and G. Huang, RSC Adv., 2015, 5, 88214 RSC.
- (a) K. Xu, Y. Fang, Z. Yan, Z. Zha and Z. Wang, Org. Lett., 2013, 15, 2148 CrossRef CAS PubMed; (b) L. Xiang, Y. Niu, X. Pang, X. Yang and R. Yan, Chem. Commun., 2015, 51, 6598 RSC; (c) B. Zhang, C. Wan, Q. Wang, S. Zhang, Z. Zha and Z. Wang, Acta Chim. Sin., 2012, 70, 2408 CrossRef CAS; (d) H. Huang, X. Ji, W. Wu and H. Jiang, Adv. Synth. Catal., 2013, 355, 170 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra08174f |
| This journal is © The Royal Society of Chemistry 2016 |