Towards the development of uniform closed cell nanocomposite foams using natural rubber containing pristine and organo-modified nanoclays
Ali Vahidifara,
Saied Nouri Khorasania,
Chul B. Parkb,
Hossein Ali Khonakdar*cd,
Uta Reuterc,
Hani E. Naguibb and
Elnaz Esmizadehe
aDepartment of Chemical Engineering, Isfahan University of Technology, 84156-83111, Isfahan, Iran
bDepartment of Mechanical and Industrial Engineering, University of Toronto, M5S 3G8, Toronto, Canada
cDepartment of Processing, Leibniz Institute of Polymer Research Dresden, D01069, Dresden, Germany
dDepartment of Polymer Engineering, Faculty of Process, South Tehran Branch, Islamic Azad University, P.O. Box 19585-466, Tehran, Iran. E-mail: khonakdar@gmail.com; Tel: +98-21-88950931
eDepartment of Polymer Engineering, Bonab University, 5551761176, Bonab, Iran
First published on 27th May 2016
Abstract
A closed cell rubber foam, based on a natural rubber (NR)/nanoclay nanocomposite, was produced using a one-step foaming process with compression molding. The effects of three different nanoclays on the properties of the NR/nanoclay nanocomposite foam were examined: an un-modified nanoclay (Cloisite Na+) and two organo-modified nanoclays (Cloisite 20A and Cloisite 30B). We examined their curing behavior, foam morphology, sound absorption, and their mechanical and thermal properties. The morphological results from X-ray diffraction (XRD) and Transmission Electron Microscopy (TEM) showed that the Cloisite Na+ agglomerated in the NR matrix while an intercalated-exfoliated/fully-exfoliated morphology was seen in the Cloisite 30B and the Cloisite 20A, respectively. The rheometry results showed that all three nanoclay types increased the NR's curing rate, and also accelerated its scorch and curing time. Other results showed that the organo-modified nanoclays, which were the ammonium-salt modified nanoclays (Cloisite 30B and Cloisite 20A), improved the foam's curing behavior more than the pristine Cloisite Na+. Using the one-step foaming process kept the nanocomposite foam density and expansion ratio constant in all of the samples, independent of the nanoclay type. Meanwhile, the scanning electron microscopy (SEM) results showed that the nanoclay increased the cell density and decreased the cell size, depending on the nanoclay type. The mechanical properties of NR nanocomposite foams including the hardness and the modulus were improved by incorporating the nanoclays. At the same time, there was a gradual deterioration in the foams' sound absorption and thermal stability behavior.
Introduction
Polymer–clay nanocomposites are currently receiving a great deal of attention. This is because of the fact that adding only a small fraction of clay to the polymer matrix significantly improves their properties. The ways in which nanoclay has improved different kinds of polymers, such as thermoplastics,1,2 thermoset polymers,3 rubbers4,5 and their foams,6–10 have been well documented in the literature. Research has shown that the nanoclay improves the physical, mechanical and thermal properties, as well as gas barrier behavior when there is a satisfactory nanoclay dispersion in the polymer matrix. With respect to the effects of the different nanoclays on solid natural rubber (NR),11–13 it was found that an organo-modified clay (or organoclay) had better dispersion as well as a faster curing rate and a higher curing density and modulus when compared with the unmodified nanoclay and pure NR.14,15Plastic foams have long been commonly used in scientific studies and industrial applications,16–18 while it is only recently that rubber foams have begun to attract interest. A literature review showed that rubber foams have been successfully prepared using a two-step foaming process based on NR,19–21 poly(ethylene propylene diene) (EPDM),22–24 acrylonitrile butadiene rubber (NBR),24,25 chlorinated polyethylene rubber (CPE),26 chloroprene rubber (CR)27 and styrene-butadiene rubber (SBR).28,29 One can conclude from these studies that two kinds of parameters control rubber foam properties as well as rubber foam morphology. These include: (a) processing parameters such as the foaming temperature and pressure, (b) the formulation parameters such as the fillers, the blowing agent, and the curing system.
Ariff et al.30 showed that the grade of the rubber had an important effect on the NR foam prepared via the two-step foaming process. Their results showed that the grade of the rubber, which had a higher cure rate and crosslink density, produced a foam with smaller cells, higher cell density, and improved properties. The relationship between the content of the chemical blowing agent and the foam morphology has been reported for NR and EPDM foams in the two-step foaming process.22,31,32 There was a gradual reduction in the crosslink density, foam density and also in the mechanical properties when the foaming agent content was increased. The effect of filler content on NR closed cell foams' morphology and properties has been previously studied.22,30,33 The results showed that the foam density and, consequently, the mechanical properties of foams were increased by increasing the filler content. In our previous study, we proposed a novel one-step foaming process.34 This method featured a constant expansion ratio and foam density along with controllable cell density and mechanical properties.
Because there are no studies in the literature that assess the fabrication of natural rubber/nanoclay nanocomposite foams, our work has aimed to address this by finding a successful way to prepare the foam. For the first time, we applied our recently developed one-step foaming process with compression molding to fabricate NR/clay nanocomposite foams. Again, for the first time, we studied how nanoclay influences the curing behaviour, the foam morphology, and both the mechanical and the sound absorption properties of NR nanocomposite foams. Our research provides valuable data on the microstructure of elastomer foams in the presence of pristine and organo-modified nanoclays. This knowledge base supports a detailed structure from which properties can be established for uniform nanocomposite systems that contain these types of nanofillers. We have discussed in depth the correlation between the nanoclay's dispersion states and the NR foams' properties. In the present study, NR/clay nanocomposite foams were fabricated using our recently developed one-step foaming process with compression molding. We used morphological techniques to investigate the foams' cell morphology in the presence of nanoclay having various dispersion states. The mechanical, thermal and sound-absorption properties of the NR/nanoclay foams in the one-step process were examined. We have discussed in depth the correlation between the nanoclay's dispersion states and the NR foams' properties.
Experimental
Materials
Standard Malaysian NR (SMR) with a trade name of SMR-20 (Mooney viscosity of ML (1 + 4)@100 °C = 55) was obtained from the Malaysian Rubber Co., Malaysia. Layered nanoclay including unmodified (Cloisite Na+) and ammonium salt modified nanoclay (Cloisite 30B and Cloisite 20A) were supplied from Southern Clay Company, USA. Sulfur, paraffin oil, zinc oxide, stearic acid, and mercaptobenzothiazole disulfide (MBTS) were of commercial grades. The chemical blowing agent azodicarbonamide (ADC) was provided by the Letai Chemical Plant (Beijing, China).Compounding and foam preparation
Before compounding, the nanoclays were kept in the oven at 80 °C for 8 h to eliminate any trace of water. An open two-roll mill was used for compounding at an ambient temperature. NR was masticated in a two-roll mill for 1 min prior to mixing. After sufficient mastication, the nanoclay was carefully added and the compounds were milled for 2 min. Other compounding ingredients, except for the curing agent, were then fed gradually into the mill and the compounding continued for 2 min. At the end, the curing agent was added and the compounding continued for an other 2 min. Table 1 shows the compounding formulations. Nanocomposite foam samples were produced via the one-step foaming process with compression molding using an electrically heated press. Cylindrical specimens, 5 cm in diameter and 1.2 cm thick, were prepared in stainless steel molds, and were compressed for 30 min at 160 °C and 50 kPa. To fix the cell structures, the mold was cooled to 25 °C and was then opened. To achieve foams of equal density (at 0.471 g cm−3) and with an expansion ratio at 2.75, 11.1 g of each compound was placed into the mold. As previously noted, in this method it becomes possible to prepare foams of equal density, independent of the nanoclay type and content.| Component | Sample code | |||
|---|---|---|---|---|
| F.0 | F.30B | F.20A | F.Na | |
| a All the contents are given in per hundred rubber (phr). | ||||
| Natural rubber (SMR20) | 100a | 100 | 100 | 100 |
| Nano clay | 0 | 3 Cloisite 30B | 3 Cloisite 20A | 3 Cloisite Na+ |
| Sulphur | 2.5 | 2.5 | 2.5 | 2.5 |
| Zinc oxide | 4 | 4 | 4 | 4 |
| Stearic acid | 1.5 | 1.5 | 1.5 | 1.5 |
| Oil | 2 | 2 | 2 | 2 |
| MBTS | 2 | 2 | 2 | 2 |
| ADC | 4 | 4 | 4 | 4 |
| Carbon black | 10 | 10 | 10 | 10 |
Characterization
The dispersion of the nanoclay was studied by using transmission electron microscopy (TEM) and small angle X-ray scattering (SAXS). The TEM images were taken in a TEM EM LIBRA120 (Carl Zeiss Germany) microscope with an accelerating voltage of 120 keV. Ultra-thin sections of about 60 nm were cut by means of a diamond knife in an ultra-microtome at −160 °C and mounted in the TEM. The SAXS patterns were collected from a D500 diffractometer (Siemens, Germany), using Ni-filtered Cu Kα radiation (λ = 0.1542 nm) at 40 kV and 35 mA. The samples were scanned in step mode for 1.5° min−1 at a scan rate in the range of 2θ < 12°.The compounds' cure characteristic was assessed using an oscillating disc rheometer (ODR-4308) at 160 °C. The cellular structure images of the foam cells were obtained using a scanning electron microscope (SEM) (JSM-6060, JEOL, USA). The parameters of the cells, including the cell size and the cell size distribution were manually traced from the SEM micrographs using Image J software. The SEM, samples were cut by a blade at 25 °C, and then sputter-coated with a thin layer of platinum. To study the nanoclay's effect on the NR foams' mechanical properties, a compression test was performed using Instron-8511 material testing equipment (according to ASTM D412). The hardness (Shore A) of the foamed samples was measured by a Zwick-3100 hardness tester, Germany, (according to ASTM D2240). The rebound resilience data were determined using a Frank GMBH elasticity tester, Germany, (according to ASTM D1054). The foams' acoustic absorption behavior was studied in accordance with ASTM E1050 in a frequency range of 800–6300 Hz, using two-microphone impedance tubes, Type SW 466 (BSWA Technology Co., Ltd., China). In all of these studies, five samples were tested in each formulation and all of the samples were examined at the room temperature. Thermo gravimetric analysis (TGA) was carried out to estimate the thermal stability of the foams using a Q50 TGA (TA Instruments) in the temperature range of 25 to 650 °C under nitrogen atmosphere at the heating rate of 10 °C min−1.
Results and discussion
Nanoclay dispersion
Fig. 1 shows the XRD pattern of the nanoclays' particles and their related NR/nanoclay nanocomposites. The Cloisite Na+, Cloisite 30B, and Cloisite 20A showed diffraction peaks at 2θ = 7.45°, 2θ = 5.13° and 2θ = 3.57°, which corresponded to the basal spacing d001 = 11.87 Å, d001 = 17.23 Å and d001 = 24.75 Å, respectively (according to Bragg's law5). The net increase of basal spacing of the organoclays in the NR foam suggests that the organic modifier intercalated into the layer spacing of the unmodified nanoclay. As can be seen, there was no peak in the XRD pattern in the F.20A foam nanocomposite. It was generally accepted that the Cloisite 20A completely exfoliated in the NR matrix. The F.30B foam nanocomposite showed a small peak at 2θ = 2.4° (d001 = 36.89 Å) from which one can conclude that the Cloisite 30B had an exfoliated/intercalated morphology in the NR matrix. The peak for the Cloisite Na+ in the F.Na nanocomposite occurred at 2θ = 7.4° (d001 = 11.95 Å). It was because this type of nanoclay agglomerated in the NR matrix. | ||
| Fig. 1 X-ray diffraction spectra of the organoclay and nanocomposites filled with various type of organoclay. | ||
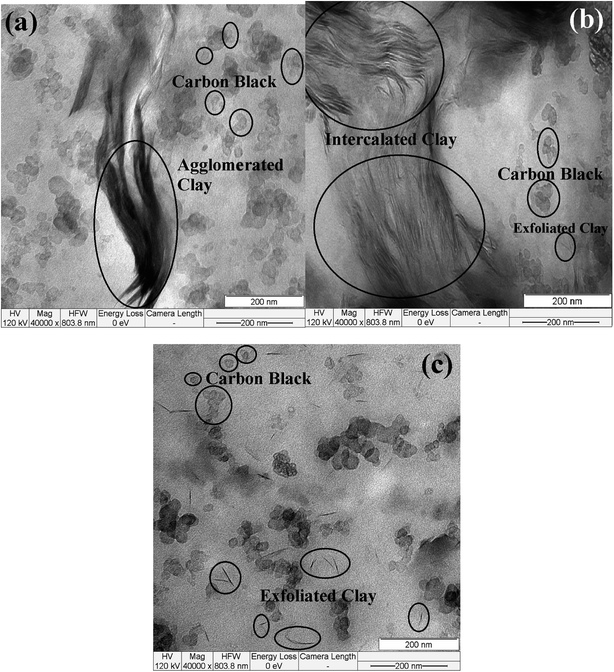
To confirm the nanoclay's dispersion in the NR foams, morphological investigations were done using TEM. Fig. 2 shows the TEM results for the nanocomposites, including the unmodified (a) and the organically modified nanoclays (b and c). The F.Na (Fig. 2a) micrograph clearly showed that the unmodified clays remained as agglomerated particles and clusters in the NR matrix. In this type of nanocomposite foam, the filler–filler interaction predominates over the filler–polymer interaction. Thus, agglomeration and non-uniformity were seen in the TEM micrograph. Fig. 2b shows that the F.30B sample had better dispersion with a heterogeneous morphology that was comprised of both exfoliated and intercalated structures. The observed partial exfoliation was in line with the XRD results, which indicated an exfoliated/intercalated morphology. This means that the polymer chains were intercalated into the interlayer space of the nanoclays to a great extent, but they were not able to completely exfoliate them. The individual random oriented clay layers separated from the intercalated tactoids were observed in the F.20A sample (Fig. 2c), which is indicative of a fully exfoliated clay structure. Thus, from the TEM images, it became obvious that the organo-modified clays expanded and exfoliated in the rubber matrix due to the intercalation of the organo-modifiers. The interlayer space of the nanoclay layers found in the SAXS result was in good agreement with the TEM observations.
Curing behavior
To achieve foams with the desired properties and morphologies, it is necessary to assess the NR/nanoclay nanocomposite foams' curing process. Fig. 3 shows the effects of various types of nanoclay on the curing behaviors of the NR foams derived from the oscillating disc rheometer measurements at 160 °C. In addition, the curing reaction parameters, such as the initial torque (Mi), the final torque (Mu), the delta torque (ΔM = Mu − Mi), the curing rate (CRI = 100 × (t90 − ts)−1),35 the scorch time (ts) and the curing time (t90) are summarized in Table 2.| Sample code | Mi (N m) | Mu (N m) | Real-M90 (N m) | Calculated-M90 (N m) | ΔM (N m) | Ts (min) | Topt (min) | CRI (% min−1) |
|---|---|---|---|---|---|---|---|---|
| F.0 | 0.94 | 5.83 | 5.34 | 5.34 | 4.89 | 3.7 | 11 | 13.7 |
| F.Na | 1.08 | 9 | 8.31 | 8.2 | 7.92 | 3.6 | 10.8 | 13.88 |
| F.30B | 1.13 | 9.8 | 8.99 | 8.93 | 8.67 | 3.2 | 10.2 | 14.3 |
| F.20A | 1.2 | 10 | 9.05 | 9.12 | 8.8 | 3 | 9.8 | 14.7 |
It can be seen that the Mi values of the samples, which relates to the chain mobility and the polymers' viscosity, were in the following sequence: Mi,F.20A > Mi,F.30B > Mi,F.Na > Mi,F.0. The increase in the Mi can be attributed to the presence of the nanoclay particles. These created physical bonding and decreased the chain mobility. This activity also seems to have been responsible for the increase in both the initial viscosity and the initial torque. As Fig. 3 shows, the sample with a fully exfoliated morphology (F.20A) exhibited the highest initial viscosity due to the maximum physical bonding achieved in this sample. The sample with agglomerated nanoclay (F.Na), which had low physical bonding with the polymer chains, presented low Mi value, which was close to that of the F.0 sample. The Mi value of the sample with the intercalated morphology (F.30B) was located between two limits (Mi of F.Na and F.20A). In addition, it was found that the ΔM (the torque difference) increased in the presence of the nanoclay. In the solid rubbers, the ΔM can be indirectly correlated to the crosslink density of the rubber while in the rubber foams it may be attributed not only to the crosslink density but also to both the foams' density and its morphology. The observed increase in the ΔM of the NR foams with the addition of nanoclay was the result of two factors: the increased curing density36 and the foams' decreased cell size. In the next section, we have provided proof of this process.
As seen in Table 2, the curing time and the curing rate of the NR/nanoclay nanocomposite foams, showed that the F.20A and F.30B samples had a shorter ts and faster CRI than the F.0 sample while those of F.Na sample remained almost unchanged. The NR/nanoclay foam nanocomposites' cure characteristics were controlled by the competition between the viscosity-increasing effect and the catalytic effect of the nanoclays. The viscosity-increasing effect was ascribed to the nanoclays' dispersion, which physically hindered the mobility of the polymer chains and prevented the cure reaction. The latter was the catalytic activity of the ammonium salt groups within the organo-modified nanoclay, which can accelerate the curing reaction.15,36 The Cloisite Na+ in the F.Na samples agglomerated and as a result the chain motion, the ts and the CRI changes were negligible compared with the F.0 sample. A decreased ts in the cases of the F.30B and the F.20A samples showed how the accelerating effect of the organo-modifier overcame the decelerating effect of the viscosity during the initial stage of the cure.
Foam morphology
As was demonstrated in our previous work,34 each sample had three different layers. These were a function of the radial distance from the specimen's wall. Only very small cells or no cells at all formed in the foam's outer layer, known as the skin layer. Farther away from the wall, the second layer, or the transition layer, produced non-uniform cells. The main part of the sample consisted of relatively uniform spherical cells, which are known as the core or the central layer.Fig. 4 shows the SEM micrographs of the NR foam nanocomposite samples. As illustrated, the presence of any type of nanoclay (Fig. 4b–d) decreased the cell size and increased the cell density, when compared with the pure NR foam (Fig. 4a). The decrease in the cell size was more pronounced in the NR foams filled by the organo-modified nanoclay: the F.30B and F.20A samples (Fig. 4c and d, respectively). To provide greater clarity, a quantitative study of the foam morphology was based on the cell density, the weight average cell size (Dw), the number average cell size (Dn), and the polydispersity index (PDI) using eqn (2)–(4), respectively, as follows:
 | (1) |
| Dw = ∑niDi2/∑niDi | (2) |
| Dn = ∑niDi/∑ni | (3) |
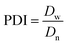 | (4) |
 | ||
| Fig. 4 Effect of nanoclay type on morphology of NR/nanoclay foams (a) F.0 (b) F.Na (c) F.30B and (d) F.20A. | ||
 | ||
| Fig. 5 Effect of nanoclay type on cell size distribution of NR/nanoclay foams (a) F.0 (b) F.Na (c) F.30B and (d) F.20A. | ||
Fig. 6 summarizes the results of the SEM quantitative analysis. The PDI commonly has a value that is equal to or greater than 1, but in foams with a uniform cell size, and isotropic mechanical properties, the PDI approaches unity. As Fig. 6 shows, the average cell size, cell size distribution, and cell density deeply depend on the nanoclay's presence and its organo-modifications. A four-fold increase occurred in the NR foams' cell density with an inclusion of unmodified nanoclay (Cloisite Na+), from 20 cell per cm3 in the F.0 sample to 80 cell per cm3 in the F.Na sample. There was a decrease to half the average cell size of the F.0 sample (with Dn = 380 and Dw = 404) to Dn = 183 and Dw = 203 in the F.Na sample. A more significant reduction in the average cell size was observed in the NR foams reinforced by the organo-modified nanoclay (that is, Dn = 160, Dw = 175 in F.30B and Dn = 160, Dw = 151 in F.20A). These results can be related to both the nucleation effect and the viscosity effect of the nanoclays. With respect to nucleation, one can surmise that the dispersed nanoclays may have acted as nucleation sites that facilitated the bubble nucleation process.8 This resulted in smaller cells and in a greater number of them. With respect to the viscosity, the nanoclay also appeared to inhibit bubble growth by affecting the rheological behavior of the matrix surrounding the growing bubbles.7
During foaming, the released gas from a blowing agent is a driving force for cell growth, while the viscosity acts as an inhibitor restricting the chain movement required for cell growth. Therefore, any parameter that helps the gas release during the foaming process increases the cell size, and any parameter that increases the movement restriction, such as viscosity and curing density (in rubbers), decreases the cell size. As we noted previously in the cure section, the nanoclay caused a considerable increase in the Mi, the Mu, and in the curing density of the NR foams, which resulted in a decreased cell size. It was proven that the organo-modification of the nanoclays more effectively dispersed them into the NR matrix. This seemed to favor nucleation, and it suggested that there were more nucleation sites available for cell growth. On the other hand, owing to the higher particle–polymer interaction that resulted from a better dispersion of the organo-modified nanoclay, these nanocomposites had a markedly improved viscosity. Such increased viscosity and curing density can also decrease the average cell size.
As noted above, the foam density was kept constant because an equal mass of each sample was put into the mold. The total volume of the foam is the sum of the volume bulk polymer and the gas bubbles. Assuming that the volume of the bulk polymer is constant in all of the samples, the total volume of the gas bubbles (Vgast) can be given as follows:
Vgast = ∑nivi ≅ n![[v with combining macron]](https://www.rsc.org/images/entities/i_char_0076_0304.gif) = cte = cte
| (5) |
![[v with combining macron]](https://www.rsc.org/images/entities/i_char_0076_0304.gif) are the number of the cells with a volume of vi, the total number of the cells, and the average volume of the foam cells, respectively. Based on the eqn (5), the reduction in the average cell size of the foam containing the organo-modified nanoclay resulted in an increased number of cells. Hence, the cell density and the number of cells per unit volume were increased, as shown in Fig. 6. In summary, a more uniform foam with a higher cell density can be obtained with NR/nanoclay foams rather than with simply NR foam.
are the number of the cells with a volume of vi, the total number of the cells, and the average volume of the foam cells, respectively. Based on the eqn (5), the reduction in the average cell size of the foam containing the organo-modified nanoclay resulted in an increased number of cells. Hence, the cell density and the number of cells per unit volume were increased, as shown in Fig. 6. In summary, a more uniform foam with a higher cell density can be obtained with NR/nanoclay foams rather than with simply NR foam.
Mechanical properties
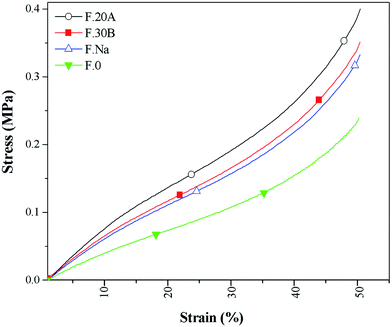
Fig. 7 shows the effect of the different nanoclay types on the compressed foams' stress–strain response. Fig. 8 shows the compressive modulus and stress at 50% strain of the NR/nanoclay nanocomposite foams obtained from the stress–strain curves. It was found from Fig. 8 that the NR/nanoclay nanocomposite foams exhibited a substantially higher compressive modulus compared with the pure NR foams. The nanoclay's enhancement effect was more pronounced in the high strains. The organo-modified nanoclay influenced the foams' mechanical behaviors more than the unmodified nanoclay. A rubber foam can be considered as a composite because it consists of two phases. These include a NR or NR/nanoclay as continuous matrix and cells (gas) as dispersed phase. Thus, the foams' stress–strain behavior depends on the mechanical properties (e.g., the modulus) and the morphology of both phases. The nanoclay increased the foams' modulus using two possible mechanisms: (1) reduction of the foam's cell size,37 and (2) increase of the matrix's modulus by improving the crosslink density and the reinforcement effect.Fig. 8 also shows the resilience of the NR/nanoclay nanocomposite foams. The NR foam had a resilience of around 74% while the unmodified nanoclay decreased the NR foams' resilience to 66%. A further reduction was observed with the inclusion of modified nanoclay of around 63% and 60% in the F.30B and the F.20A samples, respectively. Resilience is directly related to the chain mobility, and the lower resilience of the NR nanocomposite foams may have resulted from the confinement of the NR molecules by the dispersed nanoclay particles. This effect was more pronounced in the NR/modified nanoclay foam because the organo-modifier enhanced both the dispersion state (physical confinement) and the intra-gallery reaction as well as the crosslink density (chemical confinement).
The hardness of the NR nanocomposite foams versus the radial distance from the core has been plotted in Fig. 9. Based on our previous work, the inverse relationship that occurred between the hardness and the resilience was expected.34 As is shown, only with an addition of 3 wt% Cloisite Na+, the hardness of the NR foam increased from 23 to 27 shore A (approximately a 17% increase). This increase in the foams' hardness can be related to the salutary effect of the nanoclay particles on the hardness of the NR matrix and the dispersed phase (cells). They improved the hardness of the NR matrix by increasing the curing density, and the filler–rubber interaction and modulus by hindering the rubber chain mobility. Further, nanoclay can increase the foams' hardness by influencing its lower cell size. For both of these reasons, the resistance to needle penetration into the foam surface (i.e., the foams' hardness) increased. The modified nanoclay's influence on the foams' hardness was more significant than the unmodified nanoclay's. Because the modified nanoclay had a better dispersion, a higher curing density and a lower cell size, there was a greater resistance to needle penetration led to higher hardness.
It is noteworthy that the hardness of the foam was improved by increasing the distance from the center. The lower cell density near the skin resulted in a greater hardness. This behavior was pronounced in the skin layer, which had the highest foam density38–40 because there were no, or only a few, small cells in this layer.
Sound absorption
The acoustic absorption coefficient of the NR/nanoclay foams at a frequency range of 800–5800 Hz is shown in Fig. 10. The impedance tube results showed that the sound absorption coefficient in all of the samples was about 0.1, regardless of the presence of any type of nanoclay (Fig. 10a). This behavior was related to the presence of the skin layer on the sample's surface. Since the skin layer has no, or only a few, small cells, an incidental sound waves could not penetrate into the foam, and thus the most part of them were reflected. The foams' sound absorption coefficient was increased by removing the skin layer, as Fig. 10a shows. Fig. 10b illustrates the sound absorption coefficient of the skin-removed foams. Although removing the foams' skin layer improved the sound absorption coefficient in all the samples, but all of them had a small coefficient of less than 0.3. This related to a small number of open cells in the foams and the small expansion ratio (∼3). Also, the sound absorption coefficient decreased by increasing the nanoclay content. The stiff nature of the NR/nanoclay nanocomposite foams restricted the matrix's vibration-damping ability41,42 which resulted in a smaller sound absorption coefficient. Moreover, as noted earlier, adding nanoclay resulted in a smaller foam cell size, and this deteriorated the absorption performance of the foams.43–45 | ||
| Fig. 10 Sound absorption coefficient versus frequency of NR/nanoclay (a) effect of skin layer (b) effect of nanoclay type. | ||
Thermal degradation
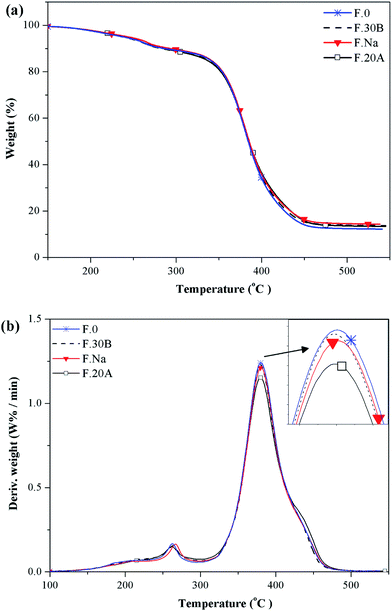
Fig. 11 shows the thermal degradation behavior of the NR/nanoclay foams. In all of the samples, the thermal degradation behavior in an inert atmosphere was expressed in a two-step process. The introduction of the nanoclay did not affect the overall degradation behavior of the NR foam. In Table 3, the thermal properties of the prepared foams are compared to highlight the nanoclay's effect on the degradation of the NR foams. The main DTG peak position and the degradation rate were found to be smaller than that of the neat NR foam. The first decomposition step occurred at 220–295 °C. It was related to the degradation of such unstable components as the chemical blowing agent, the oil, the curing system, and the other small molecular additives.22 The second degradation step occurred at 320–450 °C with a peak temperature (main DTG peak, TPeak2) of about 379 ± 1 °C, which was related to the NR degradation. As seen in Table 3, the added nanoclay had little influence on the thermal degradation properties. These results are not in concordance with previous researches, which reported that intercalated/exfoliated solid rubber (non-foamed rubber) nanocomposites had a noticeable increase in thermal stability compared with pure rubber.46 This was due to the nanoclay's contribution to the tortuosity path and to the reduction of the molecular mobility, which hindered the diffusion of the volatile decomposition products. On the other hand, in the case of the rubber foams, the nanoclay's barrier effect was negligible because the cell wall was too thin and the tortuosity pathway created by the nanoclay was insignificant. Moreover, volatile decomposition products can be trapped in cells of the foam, and this can hinder the out gassing of the volatile components.| Sample code | 1st step of weight loss | 2nd step of weight loss | Residual ash (%) | ||||
|---|---|---|---|---|---|---|---|
| TPeak1 (°C) | W1 (%) | V1 = dW1/dt (% min−1) | TPeak2 (°C) | W2 (%) | V2 = dW2/dt (% min−1) | ||
| a TPeak: maximum temperature of the DTG peak, W: weight of the sample at TPeak, V: rate of degradation at TPeak. | |||||||
| F.30B | 263.8 | 92.07 | 0.126 | 379.2 | 56.31 | 1.231 | 13.3 |
| F.20A | 261.3 | 92.61 | 0.13 | 379 | 56.46 | 1.15 | 13.3 |
| F.Na | 265.64 | 92.65 | 0.136 | 378.9 | 56.2 | 1.213 | 13.5 |
| F.0 | 263 | 93.14 | 0.14 | 379.7 | 56.1 | 1.25 | 12.04 |
Conclusion
We studied how incorporating unmodified and organo-modified nanoclays into NR foams affected their curing behavior, the foam morphology, and the mechanical, thermal and sound absorption properties. The Cloisite 20A was fully exfoliated in the NR foam. Of all the NR nanocomposite foams, the F.20A (NR/Cloisite 20A nanocomposite) sample had the highest curing rate and curing density, the smallest average cell size and the narrowest PDI (cell distribution). An improved nanoclay dispersion resulted in the highest modulus, the greatest hardness, and the lowest resilience in the F.20A sample. The Cloisite 30B showed an intercalated morphology in the NR matrix, and its mechanical improvement was less than the Cloisite 20A's. An agglomerated morphology was observed in the Cloisite Na+ in the NR matrix, and the improvement in properties was poor compared to that of the organo-modified nanoclays. A two-step degradation mechanism with negligible difference was seen in the NR/nanoclay nanocomposites. The effectiveness of the nanoclays in enhancing the NR's thermal resistance by providing a tortuous pathway diminished the foam morphology due to the thin cell walls. There was a slight decrease in the sound absorption properties, when the nanoclays were incorporated in the NR rubber.We concluded that using organo-modified nanoclays in NR foams produced interesting mechanical properties, since a uniform foam morphology and nanoclay dispersion were obtained. Therefore, a slight decrease in the sound absorption properties is compensated for by the better mechanical and morphological properties of the NR/organo-modified nanocomposite foams.
References
- M. Razavi-Aghjeh, V. Asadi, P. Mehdijabbar, H. A. Khonakdar and S. H. Jafari, Composites, Part B, 2016, 86, 273–284 CrossRef.
- S. Jafari, A. Kalati-vahid, H. A. Khonakdar, A. Asadinezhad, U. Wagenknecht and D. Jehnichen, eXPRESS Polym. Lett., 2012, 6, 148–158 CrossRef CAS.
- M. Razavi-Aghjeh, H. A. Khonakdar, S. H. Jafari, C. Zschech, U. Gohs and G. Heinrich, RSC Adv., 2016, 6, 24651–24660 RSC.
- M. Ghanbari, S. N. Khorasani, M. M. Talakesh and A. Farzadfar, J. Elastomers Plast., 2013, 45, 551–563 CrossRef CAS.
- M. Mehrabi-Mazidi, M. K. Razavi-Aghjeh, H. A. Khonakdar and U. Reuter, RSC Adv., 2016, 6, 1508–1526 RSC.
- M. Nofar, A. Ameli and C. B. Park, Mater. Des., 2015, 83, 413–421 CrossRef CAS.
- X. Cao, L. J. Lee, T. Widya and C. Macosko, Polymer, 2005, 46, 775–783 CrossRef CAS.
- W. Zheng, Y. H. Lee and C. B. Park, J. Cell. Plast., 2006, 42, 271–288 CrossRef CAS.
- J. Fu and H. E. Naguib, J. Cell. Plast., 2006, 42, 325–342 CrossRef CAS.
- X. Han, J. Shen, H. Huang, D. L. Tomasko and L. J. Lee, Polym. Eng. Sci., 2007, 47, 103–111 CAS.
- H. Sadeghi Ghari and A. Jalali-Arani, Appl. Clay Sci., 2016, 119, 348–357 CrossRef CAS.
- J. Carretero-González, H. Retsos, R. Verdejo, S. Toki, B. S. Hsiao, E. P. Giannelis and M. A. Lopez-Manchado, Macromolecules, 2008, 41, 6763–6772 CrossRef.
- R. Sengupta, S. Chakraborty, S. Bandyopadhyay, S. Dasgupta, R. Mukhopadhyay, K. Auddy and A. Deuri, Polym. Eng. Sci., 2007, 47, 1956–1974 CAS.
- A. Jacob, P. Kurian and A. S. Aprem, Int. J. Polym. Mater., 2007, 56, 593–604 CrossRef CAS.
- M. Arroyo, M. Lopez-Manchado and B. Herrero, Polymer, 2003, 44, 2447–2453 CrossRef CAS.
- C. B. Park and L. K. Cheung, Polym. Eng. Sci., 1997, 37, 1–10 CAS.
- S.-T. Lee and C. B. Park, Foam extrusion: principles and practice, CRC press, 2014 Search PubMed.
- S.-T. Lee, C. B. Park and N. S. Ramesh, Polymeric foams: science and technology, CRC Press, 2006 Search PubMed.
- M. Bashir, M. Shahid, R. Alvi and A. Yahya, Key Eng. Mater., 2012, 510–511, 532–539 CrossRef CAS.
- N. Najib, Z. M. Ariff, A. Bakar and C. S. Sipaut, Mater. Des., 2011, 32, 505–511 CrossRef CAS.
- J. Kim, J. Koh, K. Choi, J. Yoon and S. Kim, J. Ind. Eng. Chem., 2007, 13, 198 CAS.
- Z. Zakaria, Z. M. Ariff, T. L. Hwa and C. S. Sipaut, Malaysian Polymer Journal, 2007, 2, 22–30 Search PubMed.
- W. Yamsaengsung and N. Sombatsompop, Composites, Part B, 2009, 40, 594–600 CrossRef.
- A. M. Y. El Lawindy, K. M. A. El-Kade, W. E. Mahmoud and H. H. Hassan, Polym. Int., 2002, 51, 601–606 CrossRef CAS.
- W. Mahmoud, M. El-Eraki, A. El-Lawindy and H. Hassan, J. Phys. D: Appl. Phys., 2006, 39, 541–546 CrossRef CAS.
- B. S. Zhang, X. F. Lv, Z. X. Zhang, Y. Liu, J. K. Kim and Z. X. Xin, Mater. Des., 2010, 31, 3106–3110 CrossRef CAS.
- E. Bardy, J. Mollendorf and D. Pendergast, J. Phys. D: Appl. Phys., 2005, 38, 3832–3840 CrossRef CAS.
- S. S. Choi, B. H. Park and H. Song, Polym. Adv. Technol., 2004, 15, 122–127 CrossRef CAS.
- C. Nah, W. D. Kim and W. Lee, Korea Polym. J., 2001, 9, 157–163 CAS.
- Z. Ariff, Z. Zakaria, L. Tay and S. Lee, J. Appl. Polym. Sci., 2008, 107, 2531–2538 CrossRef CAS.
- N. Najib, Z. Ariff, N. Manan, A. Bakar and C. Sipaut, J. Phys. Sci., 2009, 20, 13–25 CAS.
- N. Sombatsompop and P. Lertkamolsin, J. Elastomers Plast., 2000, 32, 311–328 CrossRef CAS.
- J.-H. Kim, K.-C. Choi and J.-M. Yoon, J. Ind. Eng. Chem., 2006, 12, 795–801 CAS.
- A. Vahidifar, S. Nouri Khorasani, C. B. Park, H. E. Naguib and H. A. Khonakdar, Ind. Eng. Chem. Res., 2016, 55, 2407–2416 CrossRef CAS.
- A. Shokrzadeh, G. Nadderi and E. Esmizadeh, Fibers Polym., 2014, 15, 1694–1700 CrossRef CAS.
- M. López-Manchado, M. Arroyo, B. Herrero and J. Biagiotti, J. Appl. Polym. Sci., 2003, 89, 1–15 CrossRef.
- K. C. Rusch, J. Appl. Polym. Sci., 1969, 13, 2297–2311 CrossRef CAS.
- C. B. Park, A. H. Behravesh and R. D. Venter, Polym. Eng. Sci., 1998, 38, 1812–1823 CAS.
- C. B. Park, L. Yuejian and H. E. Naguib, Cell. Polym., 1999, 18, 367–384 CAS.
- H. Ismail and S. Suryadiansyah, J. Reinf. Plast. Compos., 2004, 23, 639–650 CrossRef CAS.
- S. Ghaffari Mosanenzadeh, H. E. Naguib, C. B. Park and N. Atalla, J. Polym. Sci., Part B: Polym. Phys., 2014, 52, 1002–1013 CrossRef CAS.
- L. Dib, S. Bouhedja and H. Amrani, Adv. Mater. Sci. Eng., 2015, 2015 DOI:10.1155/2015/896035.
- R. K. M. Chu, H. E. Naguib and N. Atalla, Polym. Eng. Sci., 2009, 49, 1744–1754 CAS.
- D. Jahani, A. Ameli, P. Jung, M. Barzegari, C. B. Park and H. E. Naguib, Mater. Des., 2014, 53, 20–28 CrossRef CAS.
- D. Jahani, A. Ameli, M. Saniei, W. Ding, C. B. Park and H. E. Naguib, Macromol. Mater. Eng., 2015, 300, 48–56 CrossRef CAS.
- T. P. Mohan, J. Kuriakose and K. Kanny, J. Ind. Eng. Chem., 2011, 17, 264–270 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |