A sepiolite-based united cross-linked network in a soybean meal-based wood adhesive and its performance
Abstract
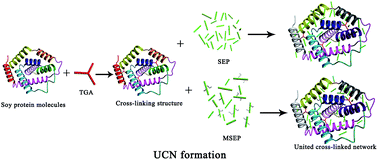
In this study, soybean meal and triglycidylamine (TGA) were used to develop soybean meal-based adhesives (denoted as SM adhesive and TSM adhesive). The sepiolite (SEP) was initially treated with KH-560 and then introduced into the soybean meal-based adhesive system to form a united cross-linked network (UCN) to compliment the water resistance of the resultant adhesive. Three-ply plywood was fabricated to measure the wet shear strength of the adhesive. The functional groups, cross section, crystallization property and degree of crosslinking of the resultant adhesives were characterized in detail. The experimental results showed that modified SEP (MSEP) could distribute well in the TSM adhesive system and improved the water resistance of the adhesive by 45.7%. The epoxy groups on the MSEP combined the cross linked protein network with the SEP system forming a UCN that increased the degree of crosslinking in the adhesive, which greatly improved the adhesive's water resistance. This UCN also produced a synergistic toughening effect and created a compact fracture to prevent moisture intrusion, which aided water resistance. The wet shear strength of the resultant plywood bonded by the TSM/MSEP adhesive with the UCN was improved by 228% (1.18 MPa) over that of the native SM adhesive, which met the interior use plywood requirement.

 Please wait while we load your content...
Please wait while we load your content...