An in situ confinement strategy to porous poly(3,4-ethylenedioxythiophene)/sulfur composites for lithium–sulfur batteries†
Abstract
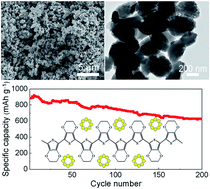
Lithium–sulfur (Li–S) batteries are receiving intense interest because of their high theoretical energy density and low cost. However, the rapid capacity fading is a significant problem facing the application of Li–S batteries. Herein, we describe an in situ confinement strategy for preparing a porous poly(3,4-ethylenedioxythiophene)/sulfur (pPEDOT/S) composite for Li–S batteries. The as-prepared pPEDOT/S composite exhibits a monodispersed nanostructure with sizes in the range of 400–600 nm. The pPEDOT/S composite electrode exhibits excellent cycling stability and high specific capacity. At a current rate of 0.5C, the pPEDOT/S electrode exhibits a high specific capacity of 883 mA h g−1 and a capacity retention of 71% after 200 cycles. During the charge/discharge process, the porous nanostructure could facilitate rapid electrolyte diffusion and accommodate the volumetric expansion. The chemical interaction between the PEDOT and polysulfides and discharged products could efficiently avoid the dissolution of polysulfides and the irreversible deposition of discharged products. The unique nanostructure plus the excellent electrochemical performances of the composites described in the current study allow for new opportunities to design high-performance electrodes for Li–S batteries.

 Please wait while we load your content...
Please wait while we load your content...