Side-chain-type quaternized naphthalene-based poly(arylene ether ketone)s for anhydrous high temperature proton exchange membranes
Guibin Lia,
Chengji Zhao*ab,
Chongyi Zhua,
Chunyu Rua,
Xuefeng Liab,
Duo Qia,
Yuxue Weia and
Hui Naab
aAlan G. MacDiarmid Institute, College of Chemistry, Jilin University, Changchun 130012, PR China. E-mail: zhaochengji@jlu.edu.cn; Fax: +86 431 85168870; Tel: +86 431 85168870
bKey Laboratory of Advanced Batteries Physics and Technology (Ministry of Education), Jilin University, Changchun 130012, PR China
First published on 10th October 2016
Abstract
Novel side-chain-type naphthalene-based poly(aryl ether ketones) with quaternary ammonium groups (Q-SCT-NPAEK) were synthesized and doped with phosphoric acid (PA), which can be used as potential high temperature proton exchange membranes. The PA doped Q-SCT-NPAEK membranes showed high thermal stabilities and high glass transition temperatures (Tg > 228 °C). They all have high tensile strengths, which were higher than 72 MPa. The introduction of a naphthalene moiety greatly improves the PA doped membranes' mechanical and thermal stabilities, which are much better than the commonly reported PA doped polybenzimidazole membrane. PA doped Q-SCT-NPAEK-100% with the highest IEC exhibits the highest weight uptake (635 wt%), doping level (16.6) and proton conductivity (46 mS cm−1 at 160 °C). Interestingly, these PA doped membranes still possess high thermal and mechanical stabilities, reaching up to 285 °C (T5%) and 15 MPa, respectively.
Introduction
As the environmental pollution and energy shortage is becoming worse, clean and efficient renewable energy sources have been urgently required. In recent years, proton exchange membrane fuel cells (PEMFCs) have gained much attention as promising power devices for renewable energy, because of their low emission of pollutants, high efficiency and energy density.1,2 As one of them, high-temperature PEMFCs (HT-PEMFCs) that operate above 80 °C under low-humidity have attracted extensive research interest over the last few decades.3–5 Compared with low-temperature PEMFCs, they have several significant advantages, including the utilization of high CO-rich reformed hydrogen, the simplified water and heat management, the less demand for coolants and the reducing usage of precious catalysts.6,7 As the critical components of HT-PEMFCs, the ideal proton exchange membranes (PEMs) require high proton conductivity, good mechanical and dimensional stability at low humidity condition.8–10 So far, phosphoric acid (PA) doped polybenzimidazole (PA/PBI) membranes have been widely investigated as potential PEMs for HT-PEMFCs, owing to their excellent mechanical and thermal stabilities induced by intermolecular hydrogen bonds.11 However, the intermolecular hydrogen bonds always lead to bad solubility or even insolubility for high molecular weight PBI. Furthermore, to achieve a high proton conductivity for these PA/PBI membranes usually requires a high acid doping level. Unfortunately, at a high acid doping level, it suffered from the significant loss of mechanical strength.12–15 Moreover, the serious pollution in the synthesis process of PBI has also restricted its wide-spread application.16 Therefore, these disadvantages have greatly stimulated the research of alternative. PEM materials with high PA doping level and sufficient mechanical strength.17,18Other alternative materials for HT-PEMs are thermo-stable polymer electrolytes, such as aromatic poly(ether ketone/sulfone)s containing N-heterocyclic or quaternary ammonium groups on the main-chains or side-chains. For example, Kallitsis's group reported a series of aromatic poly(arylene ether)s containing pyridine units on the main-chain for high temperature fuel cell applications.24 They showed good film forming ability, excellent mechanical and thermal stabilities. The PA doping ability of these polymer electrolytes depends on the contents of basic groups and detailed chemical structure.19–23 Our groups have synthesized a series of poly(arylene ether ketone)s with quaternary ammonium groups on the main chain. The quaternary ammonium groups have a good bonding ability with PA molecules, thus resulting in a high PA doping level. Hence, a high proton conductivity up to 56.9 mS cm−1 was obtained for this membrane in the anhydrous state at 200 °C.18 The results demonstrated that the PA doped alkaline ion clusters are responsible for such a high proton conductivity. However, there are still few reports on the side-chain-type quaternary ammonium functionalized copolymers and the corresponding PA doped membranes with high ionic exchange capacities (IEC).
In this work, a series of side-chain-type quaternary ammonium functionalized naphthalene-based PAEK (Q-SCT-NPAEK) was prepared from an epoxide ring-opening reaction between hydroxyl-naphthalene PAEK (HNPAEK) and 2,3-epoxypropyltrimethylammonium chloride (EPTAC). The quaternary ammonium groups on the flexible side chains could guarantee a good PA doping ability and high proton conductivity. Meanwhile, the introduction of naphthalene moiety on the polymer backbone could increase the stiffness of polymer chain and improve the mechanical and thermal stabilities of PA doped membranes. It is supposed that the PA doped Q-SCT-NPAEK membranes exhibit high proton conductivity, good thermal and mechanical stabilities at elevated temperature and anhydrous conditions. The detailed properties of the undoped and doped membranes were evaluated in this work, such as IEC, PA doping level, dimensional change, and proton conductivity. The thermal and mechanical properties of these membranes were also studied by dynamic mechanical analysis (DMA), thermal gravimetric analysis (TGA) and tensile measurement in detail.
Experiment
Materials
The monomer 1,5-bis(4-fluorobenzoyl)-2,6-dimethoxynaphthalene (DMNF) was prepared via a Friedel–Crafts acylation according to our previous work.25 Dimethyl sulfoxide (DMSO), potassium carbonate, N-methyl-2-pyrrolidone (NMP), toluene and phosphoric acid solution (85 wt%, 14.6 mol L−1) were purchased from Beijing Fine Chemicals Co Ltd. 4,4′-Difluorodiphenylmethanone (DFDP), hydroquinone and glycidyl trimethyl ammonium chloride (EPTAC) were purchased from Aladdin Co Ltd. The PBI were lab made according to our previous work. The intrinsic viscosity (ηsp/c) was measured from polymer solution of concentrated sulfuric acid (H2SO4). The viscosity of PBI is 0.88 dL g−1, indicating a high molecular weight. All these solvents and reagents were reagent grade and were used as received.Synthesis of polymers
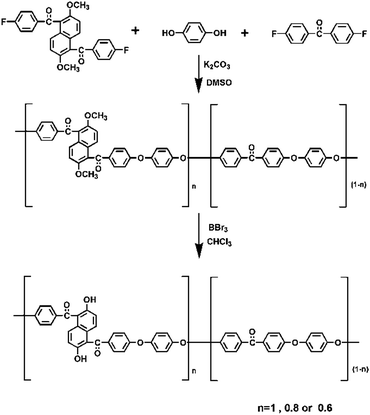
As shown in Scheme 1, dimethoxynaphthalene poly(aryl ether ketone) (MNPAEK) and dihydroxynaphthalene poly(aryl ether ketone) (HNPAEK) copolymers were synthesized according to our previous work.25 Taking MNPAEK-80% and HNPAEK-80% (the mole ratio of DMNF to DFDP is 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) as an example, the synthesis procedure is briefly described as follows.
20) as an example, the synthesis procedure is briefly described as follows.
MNPAEK-80%: samples of hydroquinone (0.01 mol, 1.1 g), K2CO3 (0.01 mol, 1.38 g), DMNF (0.08 mol, 3.46 g), DFDP (0.02 mol, 4.36 g), toluene (10 mL) and DMSO (16 mL) were added into a 250 mL three-neck flask equipped with a Dean–Stark trap and a nitrogen inlet/outlet. Under mechanical stirring, the solution was heated to 130 °C for about 2 h until the water was removed completely. Then the temperature was increased slowly to 180 °C and kept for about 4 h, until a highly viscous mixture was obtained. The resulted copolymer was poured into DI water, washed several times and then dried in vacuum at 100 °C for 24 h.
HNPAEK-80%: a sample of MNPAEK-80% (2 g) was dissolved into 20 mL dichloromethane. The solution was kept at 0–5 °C in the ice bath and 2 M BBr3 solution in dichloromethane was then added dropwise. After stirring at room temperature for 12 h, the mixture was poured into ice-water to quench excess BBr3. The resulted copolymer was washed with methanol and water, dried in vacuum at 100 °C for 24 h.
Q-SCT-NPAEK-80%: samples of HNPAEK-80% (3.38 mmol hydroxyl groups, 0.8 g) and EPTAC (3.12 mmol, 0.39 g) were dissolved into 30 mL DMSO at room temperature and stirred for 10 minutes under nitrogen atmosphere. After keeping the reaction at 80 °C for 6 h, the viscous solution was precipitated into 200 mL of acetone and washed with methanol several times. The filtrated powder was dried in vacuum at 50 °C for 12 h. The synthesis procedure is shown in Scheme 2.
Preparation of membranes
The membranes were prepared by casting 10 wt% Q-SCT-NPAEK-xx (xx: the mole percent of DMNF) solutions in DMSO onto clean glass plates. After drying at 60 °C for 12 h, the membranes were dried at 80 °C in vacuum for another 24 h to remove any excess of the solvent. The acidification of the membranes was performed by immersing the membranes into 14.6 M H3PO4 solution at 70 °C for 24 h and washed with deionized water to remove the residual acids. The dried acid form membranes were obtained by drying them at 80 °C for 12 h. The thickness of these obtained membranes was in the range of 70–90 μm.Characterization and measurements
1H NMR spectrum were measured on a 500 MHz Bruker Avance 510 spectrometer using tetramethylsilane (TMS) as the standard and DMSO-d6 as the solvent. The TGA measurements were carried out on a Perkin-Elmer TGA-1 thermo-gravimetric analyzer. Before the measurements, all the membranes were preheated in a vacuum oven at 100 °C 24 h to remove the solvent and water completely, and then the membranes were heated at a heating rate of 10 °C min−1 under nitrogen atmosphere from 100 °C to 700 °C. The Fourier-transform infrared (FTIR) spectroscopy of dried membrane samples was recorded on the power samples dispersed in dry KBr in the form of disks, using a BRUKER Vector 22 FTIR spectrometer at a resolution of 4 cm−1. DMA measurement was performed on a TA instrument (DMA Q800) in tension mode on films. The tests were carried out with a fixed frequency of 1 Hz, 1 N applied pre-force and an oscillation amplitude of 10 μm. The storage modulus (E′) and loss modulus (E′′) were obtained at a heating rate of 5 °C min−1 in air. The mechanical properties of membranes were measured at room temperature and room humidity on a tensile machine (SHIMADZU AG-I 1 kN) at a strain rate of 2 mm min−1. At least five samples (15 mm × 4 mm) were used for each test and their average values were calculated.Weight uptake, PA doping level and dimensional change of membranes
The doping level, dimensional change, weight uptake and water uptake were recorded as the weight increasing for these Q-SCT-NPAEK-xx membranes before and after doping with PA. Each Q-SCT-NPAEK-xx membrane was cut into 5 cm × 5 cm, dried at 150 °C in vacuum oven for 24 h and their weights (WD) were recorded. PA uptake was calculated by the difference between weight uptake and water uptake. After immersing into a 14.6 M PA solution at 70 °C for 24 h, the membrane was taken out, wiped the PA solution on the surface and weighted (WW), then dried in vacuum oven at 80 °C to remove water. The weight of anhydrous PA doped membrane was recorded as WA. The weight uptake, water uptake and PA doping level was calculated by eqn (1), (3) and (4), respectivity. Dimensional increase was measured by the length change in the plane direction and calculated by eqn (2). LU and LW are the lengths of dried undoped membrane and undried doped membrane, respectively. MWPA is the mole weight of PA. The corresponding equations are listed as follows:
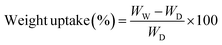 | (1) |
 | (2) |
 | (3) |
 | (4) |
| PA uptake = weight uptake − water uptake | (5) |
The proton conductivity (σ) and IEC
Each membrane coupon (size: 1 cm × 4 cm) and the thicknesses were measured. The proton conductivity (σ) of anhydrous PA doped membrane was measured with a Princeton Applied Research Model 2273 potentiostat/galvanostat/frequency response analyzer by a four-probe AC impedance method from 0.1 Hz to 100 kHz. The proton conductivity was calculated by the following equation:
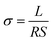 | (6) |
The IEC of Q-SCT-NPAEK-xx membranes was measured as follow: each sample was immersed into 40 mL NaOH aqueous solution (0.01 M) for two days. Then the membranes were taken out and the solution was titrated with 0.01 M HCl aqueous solution. The consumed volume of HCl aqueous solution was recorded. The membranes were dried at 80 °C for 24 h and their weights were measured. The IEC was calculated by the following equation:
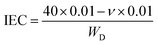 | (7) |
Results and discussion
Synthesis of Q-SCT-NPAEK copolymers
HNPAEK copolymers containing reactive hydroxyl groups was synthesized by a demethylation reaction from methoxyl-containing MNPAEK, which was synthesized by an aromatic nucleophilic polycondensation of DMNF, DFDP and hydroquinone as shown in Scheme 1. The hydroxyl contents of HNPAEK copolymers (HNPAEK-xx, xx: the mole percent of DMNF) were controlled by varying the feed ratios of DMNF and DFDP. Then, the Q-SCT-NPAEK copolymers were derived from an epoxide ring-opening reaction between the hydroxyl groups of HNPAEK-xx and the epoxy groups of EPTAC. Fig. 1 shows the 1H NMR spectra of HNPAEK-100% and the corresponding Q-SCT-NPAEK-100%. As shown in Fig. 1(a), the chemical shift at 9.8 ppm is attributed to the hydroxyl (–OH) functional group of HNPAEK. After the quaternary ammonium groups were grafted onto the side chains of HNPAEK by an epoxide ring-opening reaction with EPTAC, the peak for –OH group at 9.8 ppm disappeared in the spectrum of Fig. 1(b). The new peaks appearing at about 2.91 ppm and 4.49 ppm were assigned to the methyl protons on the quaternary ammonium groups and the methylene protons on the side chains.Fig. 2 shows the FTIR spectra of HNPAEK and Q-SCT-NPAEK-xx copolymers. After grafting quaternary ammonium groups, the spectra of Q-SCT-NPAEK-xx demonstrated several characteristic absorption bands at 1370 cm−1 assigning to the C–H bond deformation vibration of quaternary ammonium groups. Simultaneously, the asymmetric and symmetric stretch vibrations of the whole quaternary ammonium group appeared at 951 and 750 cm−1. The infrared band located at 3440 cm−1 for HNPAEK-100% belonged to the hydroxyl groups and hydronium ions that interact with the –OH group of the polymer. For the Q-SCT-NPAEK-xx, the broad band around 3440 cm−1 decreased in its intensity, which can be attributed to the H-bond associated between the quaternary ammonium cations (–N+(CH3)3) or isopropyls after the epoxy-opening reaction of EPTAC with HNPAEK-xx. Therefore, the 1H NMR and FTIR results confirmed the successful graft of quaternary ammonium groups on the side chains.
The IEC values, weight uptake, PA doping level and dimensional change of membranes
For the Q-SCT-NPAEK membranes, the quaternary ammonium groups are the functional groups which can interact with the phosphoric acid and result in good doping ability. Q-SCT-NPAEK-xx membranes with higher IEC were prepared in order to improve the PA doping level. The IEC values of Q-SCT-NPAEK-xx membranes increased with the addition amounts of DMNF. In order to obtain the PA doped membranes and improve the doping level, these Q-SCT-NPAEK-xx membranes were immersed into PA solution (14.6 M) at 70 °C for 24 h. For comparison, the PA doped PBI membrane was also prepared at the similar condition.Table 1 lists the IEC values, weight uptake, PA doping level and dimensional change of these PA doped Q-SCT-NPAEK-xx and PBI membranes. As expected, both the total weight uptake and dimensional change of Q-SCT-NPAEK-xx membranes increased with the IEC values increasing. For example, the weight uptake and dimension change of these membranes increased from 295.4% to 635.6%, 36.4% to 97.3%, respectively, as the IEC values increased from 2.01 to 2.53 mmol g−1. It can be concluded that the Q-SCT-NPAEK membranes are favorable for the absorption of PA with a suitable dimensional change.
| Samples | IEC (mmol g−1) | Weight uptake (%) | Water uptake (%) | PA uptake (%) | Doping level | Dimension increase (%) | σa (mS cm−1) | |
|---|---|---|---|---|---|---|---|---|
| 120 °C | 180 °C | |||||||
| a Samples were tested under anhydrous conditions.b PA/Q-SCT-NPAEK-100% was broken and no proton conductivity was obtained.c PBI immersed in 14.6 M phosphoric acid solution at 80 °C for 24 h. | ||||||||
| PA/Q-SCT-NPAEK-100% | 2.53 | 635.6 | 211.8 | 423.8 | 16.6 | 97.3 | 31.1 | —b |
| PA/Q-SCT-NPAEK-80% | 2.29 | 462.7 | 148.3 | 314.4 | 13.8 | 53.6 | 19.0 | 33.7 |
| PA/Q-SCT-NPAEK-60% | 2.01 | 295.4 | 87.1 | 209.3 | 10.4 | 36.4 | 11.4 | 28.6 |
| PA/PBIc | — | 324.2 | 145.9 | 178.3 | 4.5 | 25.8 | 7.7 | 11.2 |
For these membranes, the total weight uptake was attributed to the absorption of both water uptake and PA. To investigate the influence of PA on the membrane's weight increase, PA/Q-SCT-NPAEK membranes were dried in vacuum oven to remove the water without any loss of PA. As shown in Table 1, the water uptake values of doped membranes are in the range from 87.1% to 211.8%, and it could be caused by the redundant water absorbed by PA in the doped membranes. The resulting water was an important factor that is beneficial for increasing the conductivity of PA doped membranes.
For PA/Q-SCT-NPAEK membranes, we take the definition of the doping level by the average number of PA bonded by each quaternary ammonium group. As shown in Scheme 3, PA/Q-SCT-NPAEK-xx membranes exhibited the doping level from 10.4 to 16.6, which is much higher than that of PA/PBI we prepared at the same preparation condition for comparison. The results are similar to the doping level of meta-PBI by immersing the dried membrane in PA solution, which is 13–16 of PA for each mole repeating unit of meta-PBI.27,28 The high doping level of Q-SCT-NPAEK-xx membranes can be contributed to both the increased volume fraction caused by the pendant quaternary ammonium groups on the side chain and the strong basicity of the pending quaternary ammonium groups.
Proton conductivity
For the PA doped PEMs, PA acts both as donor and accepter of protons and builds the hydrogen bond network for proton conduction. Therefore, the PA doping level is an important factor for proton conductivity. The proton conductivities of PA/Q-SCT-NPAEK-xx membranes were measured and the results are shown in Fig. 3. Not surprisingly, the proton conductivities increased with the increased temperature from 80 °C to 180 °C under anhydrous condition.The highest proton conductivity was 46 mS cm−1 for PA/Q-SCT-NOAEK-100% at 160 °C, due to its highest doping level up to 16.6, which is almost 4 times higher than that of PBI at the same PA doping condition. As shown in Table 1, the doping level of PA/Q-SCT-NPAEK-60% and PA/Q-SCT-NPAEK-80% membranes is 10.4 and 13.8, respectively, which shows an increasing tendency with the IEC of Q-SCT-NPAEK increasing. Due to the high PA doping levels, they also showed much higher proton conductivities than those of PA/PBI in the range from 80 °C to 180 °C. It can be concluded that the proton conductivity of these PA doped PEMs almost depends on the IEC values of quaternized polymers and the amount of phosphoric acids. One portion of PA was attached to the polymer by acid–base interaction in the form of ion pairs;15 the other portion of PA dispersed in the membrane by the formation of hydrogen bonds among PA molecules. The continuity of the hydrogen bonding network within the PA was crucial to the efficiency of proton hopping. For the PA/Q-SCT-NPAEK-xx membranes, the flexible side chain is beneficial for the formation of continuous hydrogen bonding network and facilitating the proton hopping.17 Therefore, the high proton conductivity is anticipated for the side-chain-type quaternized polymer membranes.
Thermal gravimetric analysis and dynamic mechanical analysis
DMA is an indispensable analytical method for polymeric material study. The E′ and the tan delta curves of Q-SCT-NPAEK-xx membranes are shown in Fig. 4a. Before the test, all the membranes were kept at 60 °C in vacuum for 24 h. It could be seen that the Tg values of membranes increase with the content of naphthalene moiety increasing. The Tg values of Q-SCT-NPAEK-60%, Q-SCT-NPAEK-80%, Q-SCT-NPAEK-100% membranes are 228, 243 and 248 °C, respectively. The introduction of naphthalene moiety in the main chains and the hydrogen-bonds formed between the quaternary ammonium cations (–N+(CH3)3) and isopropyl alcohols in the side chains might lead to a higher Tg, which is also attributed to the high temperature resistance of Q-SCT-NPAEK-xx membranes. This observation was also derived from their TGA curves. As shown in Fig. 4b and c, the temperature where the membrane loses 5% weight (T5%) is recorded and used to evaluate the thermal stability of the membrane. | ||
| Fig. 4 The DMA curves of Q-SCT-NPAEK-xx (a); TGA curves of Q-SCT NPAEK-xx (b) and PA doped membranes (c). | ||
The T5% values of Q-SCT-NPAEK-xx membranes are 295, 298 and 304 °C, respectively, which also increase with the content of naphthalene moiety and hydrogen-bonds increasing. For the PA/Q-SCT-NPAEK membranes, the first decomposition temperature was about 160 °C, which could be assigned to the loss of water produced by acid dimerization; the second and the third weight loss were considered as the loss of quaternary ammonium groups and the main chains, respectively. Furthermore, after PA doping, the T5% values of PA doped membranes are closed to the undoped membranes. Moreover, there is no visible change in the proton conductivity of these PA doped membrane after placing them in the oven at 160 °C for a week. All these results indicated that the PA/Q-SCT-NPAEK-xx membranes exhibited high thermal stabilities for application in HT-PEMFCs.
Mechanical properties
The mechanical properties of all membranes before and after PA doped were measured and their stress–strain curves are shown in Fig. 5. It can be observed that the tensile strength and the elongation at break of Q-SCT-PAEK-xx membranes are 76–104 MPa and 16–24%, respectively, which are much higher than those of PBI (Table 2). They showed a regular increasing trend with the content of naphthalene moiety increasing. The incorporation of rigid naphthalene moiety in the main chain and the formation of hydrogen-bonds in the side chains greatly improved the mechanical properties and thermal stabilities of Q-SCT-NPAEK-xx membranes. With the grafting proportion of EPTAC increasing, the content of the side-chain increased, which enhanced the flexibility of the Q-SCT-NPAEK-xx membranes. Therefore, Q-SCTNPAEK-100 shows both higher tensile strength and maximum elongation than Q-SCTNPAEK-80 and Q-SCTNPAEK-60.| Membranes | Young's modulus (MPa) | Tensile strength (MPa) | Maximum elongation (%) |
|---|---|---|---|
| a PBI was immersed in 14.6 M phosphoric acid solution at 80 °C for 24 h. | |||
| Q-SCT-NPAEK-100% | 1233.6 | 102.9 | 24.8 |
| Q-SCT-NPAEK-80% | 1289.7 | 82.7 | 15.9 |
| Q-SCT-NPAEK-60% | 1169.4 | 72.4 | 17.6 |
| PBI | 2471.3 | 71.9 | 8.8 |
| PA/Q-SCT-NPAEK-100% | 173.6 | 8.6 | 78.6 |
| PA/Q-SCT-NPAEK-80% | 199.2 | 14.1 | 72.1 |
| PA/Q-SCT-NPAEK-60% | 225.1 | 17.4 | 74.9 |
| PA/PBIa | 218.4 | 8.2 | 45.1 |
After PA doping, the tensile strengths of PA/Q-SCT-NPAEK-xx membranes were almost less than a fifth of undoped Q-SCT-NPAEK-xx membranes. Due to the plasticization effect of PA, the tensile strength decreased sharply. On the contrary, due to the plasticization, the elongation at break of PA/Q-SCT-NPAEK-xx membranes increased. The more PA the membranes absorb, the larger elongation at break and the lower tensile strength the membranes exhibit. The deterioration of mechanical stability at high H3PO4 concentration existed in all various kinds of basic polymer membranes including PA doped PBI membrane.29 However, the tensile strengths and elongation at break of PA/Q-SCT-NPAEK-xx membranes were higher than that of PA/PBI membrane, which were prepared by immersing at 80 °C in 85 wt% PA solution for 24 h. The result could be also caused by the introduction of rigid naphthalene moiety in the main chains and the hydrogen-bonds in the side chains.
Conclusions
In summary, PA/Q-SCT-NPAEK-xx membranes used as HTPEM were prepared successfully from an epoxide ring-opening reaction between HNPAEK and EPTAC. Due to the introduction of naphthalene moieties in the main chain, Q-SCT-NPAEK-xx membranes had high Tg, thermal and mechanical stabilities. What's more, the PA doped membranes still had good thermal and mechanical properties. Especially, the tensile strengths of PA/Q-SCT-NPAEK-xx membranes were comparable or even higher than PA/PBI membranes, which were both prepared by immersing in 85 wt% PA solution at 80 °C or 24 h. Both the weight uptake and the PA doping level were calculated, and it was found that the IEC values are the main factor to reflect the trend of conductivities. As the IEC increasing, the PA doping level and the proton conductivity increased. PA/Q-SCT-NPAEK-100% with a highest PA doping level of 16.6 showed the highest proton conductivity of 46 mS cm−1 at 160 °C. PA/Q-SCT-NPAEK-80% and PA/Q-SCT-NPAEK-60% membranes still showed much higher proton conductivities than PA/PBI. These results indicated that the structure of rigid moiety in the main chain and functional group in the side chain improved the proton conductivity at high temperature under anhydrous condition, while maintaining a reasonable mechanical and thermal stability at a high PA doping level. The side-chain-type PA doped Q-SCT-NPAEK-xx membranes showed promising potential to be used as high-temperature PEMs.Acknowledgements
The authors acknowledge the financial support from the Natural Science Foundation of China (No. 21474036 and 21374034) and the Fok Ying-Tong Education Foundation for Young Teachers in the Higher Education Institutions of China (142010).Notes and references
- X. Li, H. Na and H. Lu, J. Appl. Polym. Sci., 2004, 94, 1569–1574 CrossRef CAS.
- C. Wang, N. Li, D. W. Shin, S. Y. Lee, N. R. Kang, Y. M. Lee and M. D. Guiver, Macromolecules, 2011, 44, 7296–7306 CrossRef CAS.
- S. Bose, T. Kuila, T. X. H. Nguyen, N. H. Kim, K. T. Lau and J. H. Lee, Prog. Polym. Sci., 2011, 36, 813–843 CrossRef CAS.
- Y. B. Di, W. J. Yang, X. J. Li, Z. Zhao, M. R. Wang and J. M. Dai, RSC Adv., 2014, 4, 52001–52007 RSC.
- W. J. Ma, C. J. Zhao, J. S. Yang, J. Ni, S. Wang, N. Zhang, H. D. Lin, J. Wang, G. Zhang, Q. F. Li and H. Na, Energy Environ. Sci., 2012, 5, 7617–7625 CAS.
- L. Xu, H. L. Han, M. Y. Liu, J. M. Xu, H. Z. Ni, H. L. Zhang, D. Xu and Z. Wang, RSC Adv., 2015, 5, 83320–83330 RSC.
- H. Sun, Z. Sun and Y. H. Wu, Int. J. Hydrogen Energy, 2012, 37, 12821–12826 CrossRef CAS.
- Y. Shao, G. Yin, Z. Wang and Y. Gao, J. Power Sources, 2007, 167, 235–242 CrossRef CAS.
- M. R. Berber, T. Fujigaya and N. Nakashima, ChemCatChem, 2014, 6, 567–571 CrossRef CAS.
- S. J. Peighambardoust, S. Rowshanzamir and M. Amjadi, Int. J. Hydrogen Energy, 2010, 35, 9349–9384 CrossRef CAS.
- Q. F. Li, J. O. Jensen, R. F. Savinell and N. J. Bjerrum, Prog. Polym. Sci., 2009, 34, 449–477 CrossRef CAS.
- P. P. Sharma and V. Kulshrestha, RSC Adv., 2015, 5, 56498–56506 RSC.
- N. Agmon, Chem. Phys. Lett., 1995, 244, 456–462 CrossRef CAS.
- J. R. P. Jayakody, S. H. Chung, L. Durantino, H. Zhang, L. Xiao, B. C. Benicewicz and S. G. Greenbaum, J. Electrochem. Soc., 2007, 154, 242–246 CrossRef.
- A. J. Antonio, E. M. Sánchez and G. R. Pedro, Chem. Soc. Rev., 2010, 39, 3210–3239 RSC.
- D. B. Mitzi, Chem. Mater., 2001, 13, 3283–3298 CrossRef CAS.
- N. Gourdoupi, A. K. Andreopoulou, V. Deimede and J. K. Kallitsis, Chem. Mater., 2003, 15, 5044–5050 CrossRef CAS.
- W. J. Ma, C. J. Zhao, H. D. Lin, G. Zhang, J. Ni, J. Wang, S. Wang and H. Na, J. Power Sources, 2011, 196, 9331–9338 CrossRef CAS.
- D. M. Tigelaar, A. E. Palker, C. M. Jackson, K. M. Anderson, J. Wainright and R. F. Savinell, Macromolecules, 2009, 42, 1888–1896 CrossRef CAS.
- J. W. Traer and G. R. Goward, Phys. Chem. Chem. Phys., 2010, 12, 263–272 RSC.
- S. Kim, P. Kim, Y. S. Lee, S. B. Park, S. H. Lee and C. Nah, Bull. Korean Chem. Soc., 2010, 31, 1411–1414 CrossRef CAS.
- N. Zhang, B. L. Wang, C. J. Zhao, S. Wang, Y. R. Zhang, F. Z. Bu, Y. Cui, X. F. Li and H. Na, J. Mater. Chem. A, 2014, 2, 13996–14003 CAS.
- N. Zhang, B. L. Wang, Y. R. Zhang, F. Z. Bu, Y. Cui, X. F. Li, C. J. Zhao and H. Na, Chem. Commun., 2014, 50, 15381–15384 RSC.
- (a) C. I. Morfopoulou, A. K. Andreopoulou, M. K. Daletou, S. G. Neophytides and J. K. Kallitsis, J. Mater. Chem. A, 2013, 1, 1613–1622 RSC; (b) M. K. Daletou, M. Geormezi, E. K. Pefkianakis, C. Morfopoulou and J. K. Kallitsis, Fuel Cells, 2010, 10, 35–44 CAS.
- J. Zhu, K. Shao, G. Zhang, C. J. Zhao, Y. Zhang, H. T. Li, M. M. Han, H. D. Lin, D. Xu, H. B. Yu and H. Na, Polymer, 2010, 51, 3047–3053 CrossRef CAS.
- G. B. Li, C. J. Zhao, X. F. Li, D. Qi, C. Liu, F. Z. Bu and H. Na, Polym. Chem., 2015, 6, 5911–5920 RSC.
- J. A. Asensio, S. Borros and P. G. Romero, J. Electrochem. Soc., 2004, 151, 304–310 CrossRef.
- X. Glipa, B. Bonnet, B. Mula, D. J. Jones and J. Roziere, J. Mater. Chem., 1999, 9, 3045–3049 RSC.
- S. Yu, L. Xiao and B. C. Benicewicz, Fuel Cells, 2008, 8, 165–174 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |