N-Methylation of amines with methanol in a hydrogen free system on a combined Al2O3–mordenite catalyst
Jiahui Sua,
Xungang Lia,
Yunbin Chenb,
Yuancun Cuib,
Jingwei Xub,
Chao Qiana and
Xinzhi Chen*a
aKey Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: xzchen@zju.edu.cn; Fax: +86-571-87951742; Tel: +86-571-87951615
bZhejiang Jianye Chemical Co. Ltd., Hangzhou 311600, China
First published on 19th May 2016
Abstract
N-Methyl amines play a major role in the production of medicines, pesticides, surfactants and dyes. N-Methylation of primary or second amines with methanol is considered to be a green path for the synthesis of N-methyl amines and the catalyst is key. In this article, the combined Al2O3–mordenite catalyst (mass fraction of alumina is 40%) with good activity, selectivity, lifetime and stability was prepared for N-methylation of various amines with methanol in a hydrogen free system in a fixed bed reactor, and characterized by XRD, N2 adsorption and NH3-TPD. Furthermore, the methanol adsorption was investigated by in situ FTIR, and the result indicated that methoxyl species may be the active species for the N-methylation of amines.
1. Introduction
N-Methyl amines have great applications in the production of medicines, pesticides, dyes, surfactants and so on.1–3 The N-methylation reaction is an important way to synthesize N-methyl amines, and N-methylation of amines with methanol is considered to be a green production technology because its byproduct is mainly water.4For N-methylation of amines with methanol, gas–solid catalytic reactions are common methods. There are many relevant catalysts that have been reported, and they can be divided into metal-supported catalyst and non-metallic catalyst.5 Metal-supported catalyst is composed of metal active component and carrier.6 Copper-based catalysts and nickel-based catalysts are the most studied catalysts, and researchers have successfully developed some high activity and selectivity catalysts for the amination of specific alcohols by adding some other metal active component such as zinc, cobalt, and some other rare earth elements.7–9 Our co-workers have done a lot of research on metal-supported catalyst, too.7,10–14 However, selectivity is still a concern for the most amines, because the existence of metal such as nickel is easy to cause the disproportionation reaction of amines.8,15 Besides, the study of catalyst deactivation indicated that metal active component is easy to generate metal nitrides and carbides,16 so the catalyst's lifetime is not good.
The disproportionation by-products are less in non-metallic catalysts according to the literatures,17,18 hence it occurs to us that non-metallic catalyst may don't have these problems for they are solid acid without metal active components. Now researches of non-metallic catalysts are mainly concentrated in methylamine synthesis catalysts,19,20 but very few studies about other amines and alcohols, so it is worthwhile to conduct non-metallic catalysts study on other amines and alcohols.
In this article, combined Al2O3–mordenite catalysts were prepared for N-methylation of different amines with methanol in hydrogen free system in a fixed bed reactor. And reaction results shown that the activity, selectivity, lifetime and stability of the combined Al2O3–mordenite catalyst (mass fraction of alumina is 40%) were all good. The properties of the catalyst were characterized by XRD, N2 adsorption and NH3-TPD. Furthermore, in situ infrared spectra technology was introduced to study the reaction mechanism.
2. Experimental
2.1. Catalyst preparation
Combined Al2O3–mordenite catalysts were prepared by deposition–precipitation as follows: Al(NO3)3·9H2O was dissolved in the water to get an aqueous Al(NO3)3 solution (with an Al3+ concentration of 0.25 mol L−1), then a certain amount of mordenite powder (H type with Si/Al = 25, Nankai University, China) was poured into the solution under vigorously stirring. An aqueous solution of ammonia (25 wt%) was added dropwise to the above-mentioned slurry until the pH reached 7.0, and the mixture was further stirred for 2 h. The mixture was filtered and washed with deionized water, and then dried at 100 °C overnight. The solid was formed into cylindrical catalyst of 6 mm length and 4 mm diameter and finally calcined at 550 °C for 6 h, combined Al2O3–mordenite catalysts were obtained. In this article, the influence of mass fraction of alumina for the combined Al2O3–mordenite catalysts were discussed.2.2. Characterizations
XRD analysis was performed on an automated powder X-ray diffractometer system (Cu Kα radiation, 40 kV, 40 mA, PANalytical, X'Pert PRO, Holland).N2 adsorption experiment was performed using a physical adsorption instrument (Micromeritics, ASAP 2020, USA) at −196 °C. The sample was degassed under the vacuum of 3 mmHg at 300 °C for 4 h before adsorption.
NH3-TPD experiment was carried out on an autocatalytic adsorption system (Micromeritics, AutoChem II 2920, USA), which include an on-line thermal conductivity detector (TCD) and a quartz tubular reactor. Sample (0.1 g) was put in the reactor and pretreated at 500 °C with an argon flow of 30 mL min−1 for 2 h, then cooled to 80 °C. Ammonia was introduced at the flow rate of 10 mL min−1 for 30 min at 80 °C, then warming up to 100 °C to remove the physical adsorption of ammonia with the argon flow for 1 h. The temperature of reactor was programmed to increase at a ramp rate of 15 °C min−1 to 800 °C, and the amount of ammonia in effluent was measured by TCD and record as function of temperature.
Diffuse reflectance infrared spectra of absorbed pyridine for the combined Al2O3–mordenite catalyst was obtained by Agilent 660-IR Fourier transform spectrum system at 150 °C.
2.3. Catalytic reaction
The reactions of N-methylation of amines with methanol were performed in a continuous tubular reaction system (Parr 5400, USA) under the following procedure: 100 mL catalyst was placed into the stainless-steel fixed bed reactor, heated to 270 °C with a nitrogen flow of 50 mL min−1 for about 2 h until the temperature became stable, then closed nitrogen. Reaction solutions (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of methanol to amine) was added into fixed bed reactor by plunger pump (TBP 5010, China). The reaction products were analyzed by gas chromatograph (Agilent 1790F, USA) and GC-MS (Agilent 5973, USA), and external standard method was employed to make quantitative analysis. The reaction pressure was 1.0 MPa and the liquid hourly space velocity (LHSV) of reaction solution was maintained at 0.15 h−1. And the stability test of the compound catalyst was investigated at the same conditions. The flow chart of continuous tubular reaction system was shown in Fig. 1.
1 molar ratio of methanol to amine) was added into fixed bed reactor by plunger pump (TBP 5010, China). The reaction products were analyzed by gas chromatograph (Agilent 1790F, USA) and GC-MS (Agilent 5973, USA), and external standard method was employed to make quantitative analysis. The reaction pressure was 1.0 MPa and the liquid hourly space velocity (LHSV) of reaction solution was maintained at 0.15 h−1. And the stability test of the compound catalyst was investigated at the same conditions. The flow chart of continuous tubular reaction system was shown in Fig. 1.
In addition, in situ FTIR was introduced to study the reaction mechanism. For the reaction of methylamine synthesis over acidic zeolites, some researchers speculated that methanol would first generate methoxyl species, then reacted with ammonia.21–23 Inspired by this, methanol absorption on combined Al2O3–mordenite catalyst was studied using in situ diffuse reflection Fourier transform infrared spectroscopy system which contains infrared spectrometer (Agilent 660-IR, USA) and in situ suite (HARRICK, HVC, USA).
3. Results and discussion
3.1. Analysis of the combined Al2O3–mordenite catalyst
The combined Al2O3–mordenite catalysts with different mass fraction of alumina were prepared, and XRD, BET, and NH3-TPD analysis of the compound catalysts were performed.XRD analysis of the combined Al2O3–mordenite catalysts were shown in Fig. 2. Mordenite (HM) diffraction peak (2θ = 9.7°, 13.5°, 19.7°, 22.4°, 25.7°, 26.4°, 27.7°, 31.0°) was detected in all compound catalysts and its crystalline structure remained intact. Referring to literature24 and the standard XRD pattern of gamma-alumina, the diffraction peak (2θ = 36.7°, 45.5°, 66.9°) in Fig. 2 should be gamma-alumina. However, it was quite weak in both compound catalysts which indicated that gamma-alumina formed during deposition–precipitation was highly dispersed on the surface of the combined Al2O3–mordenite catalysts.
The porous structure of the combined Al2O3–mordenite catalyst were characterized by N2 adsorption and were summarized in Table 1. The surface area and pore size distribution were calculated according to the adsorption isotherm. The BET surface area decreased from 351.14 to 301.86 m2 g−1 with the increase of mass fraction of alumina from 20 to 60% and the pore volume had no obvious changes. The average pore size of the compound catalysts were gradually increasing with the increase of mass fraction of alumina. The average pore size of both compound catalysts were above 2.0 nm, which indicated that the structure of the compound catalysts belong to the mesoporous structure.
| Entry | Al2O3(wt%) | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore size (nm) |
|---|---|---|---|---|
| 1 | 20 | 351.14 | 0.26 | 4.47 |
| 2 | 40 | 338.45 | 0.29 | 4.49 |
| 3 | 60 | 301.86 | 0.28 | 4.60 |
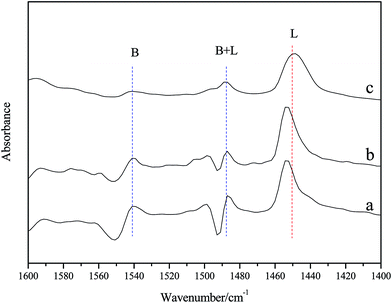
The surface acidity of the combined Al2O3–mordenite catalysts were analyzed by NH3-TPD and FTIR of adsorbed pyridine, and the profiles were shown in Fig. 3 and 4, respectively. In Fig. 4, peaks at 1540 cm−1 and 1450–1440 cm−1 are available, which means that acid sites of the combined Al2O3–mordenite catalysts contains Brönsted acid sites (at 1540 cm−1) and Lewis acid sites (at region of 1450–1440 cm−1). In addition, a band at nearly 1490 cm−1 was observed, which could be attributed to pyridine adsorbed on both Brönsted and Lewis acid sites, as well as H-boned pyridine. In Fig. 3, NH3 desorption peaks at 200 °C and 275 °C could be found, where NH3 desorption peaks of 200 °C represented the site of weak acid while the NH3 desorption peaks of 275 °C was the site of medium strong acid. From the Fig. 3, we could find that the peak area decreased with the increase of mass fraction of alumina, which demonstrated that addition of alumina reduced the acidic concentration of the compound catalysts.
The evaluation experiments of catalysts were carried out based on the reaction of n-butylamine with methanol in fixed bed reactor. The results were depicted in Table 2. Results shown that the conversion of n-butylamine decreased from 86.2 to 53.4% with the increase of mass fraction of alumina from 20 to 60% while the selectivity of N-methyl and N,N-dimethyl products was low when the mass fraction of alumina was 20%. Combined with characterization results of the catalysts, acidity of catalyst might be the reason for the difference of N-methylation reaction, and addition of alumina caused changes in the acidic concentration of the compound catalysts. The acidic concentration of the compound catalysts reduced with the increase of mass fraction of alumina, then the activity of N-methylation reaction reduced. However, the acidic concentration of the compound catalyst was too large when the mass fraction of alumina was low (20%), which were more likely to cause deamination reaction of n-butylamine to generate di-n-butylamine, then reacted with methanol to generate byproducts N,N-dibutyl-N-methylamine. Considering activity and selectivity, the combined Al2O3–mordenite catalyst with 40% mass fraction of alumina shown best performance for N-methylation reaction.
| Entry | Al2O3/wt% | Conversionb/% | Selectivity/% | ||
|---|---|---|---|---|---|
| N-Methyl butylamine | N,N-Dimethyl butylamine | N,N-Dibutyl-N-methylamine | |||
a The reactions were carried out at 270 °C temperature, 0.15 h−1 LHSV, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of methanol to n-butylamine under atmospheric pressure.b Conversion of n-butylamine. 1 molar ratio of methanol to n-butylamine under atmospheric pressure.b Conversion of n-butylamine. |
|||||
| 1 | 20 | 86.2 | 30.1 | 52.1 | 17.8 |
| 2 | 40 | 78.6 | 40.8 | 55.7 | 3.5 |
| 3 | 60 | 53.4 | 55.8 | 41.3 | 2.9 |
3.2. N-Methylation of amines with methanol
Initially, the influence of varied conditions on the reaction were investigated using N-methylation of n-butylamine with methanol to give N,N-dimethylbutylamine as model reaction, and the results were shown in Table 3. The reaction was strongly affected by temperature (Table 3, entries 1–3). The conversion of n-butylamine is low when temperature is low. The selectivity of N,N-dimethylbutylamine increased obviously as temperature increased, while it decreased at over 270 °C since a large amount of byproducts N,N-dibutyl-N-methylamine would occur. The conversion and selectivity were also significantly influenced by LHSV, because it determined the contact time with the catalyst. The conversion and selectivity both increased with the decrease of LHSV from 0.30 to 0.15 h−1 (Table 4, entries 2, 4–5). Both the conversion and selectivity were positively correlated with pressure (Table 4, entries 2, 6–7) and mole ratio of methanol to n-butylamine (Table 4, entries 2, 8–9), while they show slight increase when pressure is over 1.0 MPa, mole ratio of methanol to n-butylamine is over 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In this research, the optimal conditions were determined at 270 °C temperature, 4
1. In this research, the optimal conditions were determined at 270 °C temperature, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of methanol to n-butylamine, 0.15 h−1 LHSV and 1.0 MPa pressure.
1 molar ratio of methanol to n-butylamine, 0.15 h−1 LHSV and 1.0 MPa pressure.
| Entry | T/°C | LHSV/h−1 | P/MPa | Methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-butylamineb n-butylamineb |
Conversionc/% | Selectivityd/% |
|---|---|---|---|---|---|---|
| a The reactions were carried out in the presence of the combined Al2O3–mordenite catalyst with 40% mass fraction of alumina in hydrogen free system.b Molar ratio.c Conversion of n-butylamine.d Selectivity of N,N-dimethylbutylamine. | ||||||
| 1 | 240 | 0.15 | 1.0 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
59.1 | 58.1 |
| 2 | 270 | 0.15 | 1.0 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.0 | 87.7 |
| 3 | 300 | 0.15 | 1.0 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.4 | 76.6 |
| 4 | 270 | 0.21 | 1.0 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83.9 | 77.0 |
| 5 | 270 | 0.30 | 1.0 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
76.5 | 64.1 |
| 6 | 270 | 0.15 | 0.8 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92.8 | 73.8 |
| 7 | 270 | 0.15 | 1.2 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.2 | 89.1 |
| 8 | 270 | 0.15 | 1.0 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
69.3 | 47.5 |
| 9 | 270 | 0.15 | 1.0 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.0 | 87.9 |
| Entry | Amine | Product | Conversion/% | Selectivity/% |
|---|---|---|---|---|
a Reaction conditions: T = 270 °C, LHSV = 0.15 h−1, molar ratio of methanol/amine = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, P = 1.0 MPa. 1, P = 1.0 MPa. |
||||
| 1 |  |
 |
99.0 | 87.7 |
| 2 |  |
 |
92.0 | 78.3 |
| 3 | 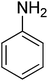 |
 |
63.5 | 57.4 |
| 4 |  |
 |
80.7 | 71.8 |
| 5 |  |
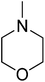 |
97.6 | 96.0 |
| 6 |  |
 |
95.1 | 91.9 |
| 7 | 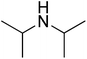 |
 |
73.6 | 95.3 |
Compared to the N-methyl technology on Co–No/γ-Al2O3 catalyst,13 the N-methyl technology in this article is with high target product yields and less disproportionation reaction. The results indicated that reaction mechanism of N-methylation with the combined Al2O3–mordenite catalyst was not as dehydrogenation/hydrogenation mechanism of metal-supported catalyst.13,25 According to literature,26 the catalytic mechanism of the combined Al2O3–mordenite catalyst might belong to dehydration mechanism, and dehydration process would be discussed in the following.
Based on the optimization of reaction conditions above, the reactions involving other amines and methanol are also investigated. The reactions were carried out in the presence of the combined Al2O3–mordenite catalyst with 40% mass fraction of alumina in hydrogen free system, and reaction results were summarized in Table 4. For fatty amines, either primary amines or secondary amines, the conversions were high (92–99%, Table 4, entries 1–2, 5–6). However, diisopropylamine gave a poor conversion, probably the steric effect of the diisopropylamine molecule have effect on the reaction (73.6%, Table 4, entry 7). Primary amine are prone to single methylation reaction to give N-methyl products, then react with methanol to give N,N-dimethyl products, so selectivity of second amines (95–96%, Table 4, entries 5, 7) was higher than primary amines (88–93%, Table 4, entries 1–2). The aromatic group had adverse effect on the conversion (Table 4, entries 3–4), and it is obvious that their conversion and selectivity is lower than aliphatic amine. Furthermore, the result of benzylamine in the N-methylation is better than aniline. Thus, it is notable that aromatic amine has less activity than aliphatic amine, and electronic effect might be the reason.
The N-methylation of n-butylamine with methanol was selected as model reaction used for the stability study of the combined Al2O3–mordenite catalyst. The stability test was performed at stable reaction conditions: 270 °C temperature, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of methanol to n-butylamine, 0.15 h−1 LHSV and 1.0 MPa, in the presence of the combined Al2O3–mordenite catalyst with 40% mass fraction of alumina in hydrogen free system. The product was analyzed at irregular time intervals over a period of 300 h and the results were summarized in Fig. 5. The conversion and selectivity fluctuated within a small range, then the constant stability of the combined Al2O3–mordenite catalyst with 40% mass fraction of alumina is concluded.
1 molar ratio of methanol to n-butylamine, 0.15 h−1 LHSV and 1.0 MPa, in the presence of the combined Al2O3–mordenite catalyst with 40% mass fraction of alumina in hydrogen free system. The product was analyzed at irregular time intervals over a period of 300 h and the results were summarized in Fig. 5. The conversion and selectivity fluctuated within a small range, then the constant stability of the combined Al2O3–mordenite catalyst with 40% mass fraction of alumina is concluded.
3.3. Methanol absorption
To better discuss the dehydration reaction process, the adsorption process of methanol on the surface of the combined Al2O3–mordenite catalyst was studied using in situ FTIR technology and result was shown in Fig. 6. The clean combined catalyst was regarded as background and the free methanol was removed by flowing N2. Initially, methanol was absorbed on the clean combined catalyst for an hour, then purging the system with nitrogen for 30 min, curve (a) was obtained. Raised to the target temperature, and curves (b–e) were gathered after purging with N2 for 30 min, respectively. | ||
| Fig. 6 Infrared spectra for methanol adsorption after purging with N2 at (a) room temperature, (b) 373 K, (c) 473 K, (d) 523 K, (e) 603 K. | ||
Room temperature adsorption of methanol results in a broad band with peaks at 2947 cm−1 and 2834 cm−1, which belong to the symmetric and asymmetric CH3 vibration of methanol. Raising the temperature to 473 K results in elimination of peak at 2834 cm−1, and new peaks at 2840 cm−1 and 2820 cm−1 appeared. When the temperature raised to 523 K, the peaks changed again. The peaks at 2947 cm−1 and 2840 cm−1 disappeared while the new peaks at 2952 cm−1 and 2846 cm−1 appeared. According to the literature,21,23 the peaks at 2952 cm−1 and 2846 cm−1 could be assigned to the symmetric and asymmetric vibrations for methoxyl species. Furthermore, the formation mechanism of methoxyl species was put forward to explain the experimental result, and shown in Fig. 7. The peaks at 2947 cm−1, 2840 cm−1 and 2820 cm−1 appeared at temperature 373 K and 473 K may be the CH3 vibration of chemisorbed methanol with its methyl group being interacted with the framework oxygen (Fig. 7, substance 2), i.e., the prestage of six-center cyclic transition state (Fig. 7, substance 3), and high temperature (more than 523 K) was needed for the dehydration of chemisorbed methanol via the transition state. At last, the N-methylation mechanism of n-butylamine with methanol on the combined Al2O3–mordenite catalyst (Fig. 7) was proposed: chemisorbed methanol was formed with the catalyst to give six-membered cyclic transition state and the transition state dehydrated to form methoxyl species, then N-methyl products were generated through the six-membered cyclic transition state which was formed by the methoxyl species and the absorbed n-butylamine or N-methylbutylamine.
4. Conclusions
In this work, the combined Al2O3–mordenite catalyst was prepared for the N-methylation of primary or second amines with methanol in hydrogen free system, and the catalyst was characterized by XRD, N2 adsorption and NH3-TPD. Catalysts evaluation experiments were carried out based on the reaction of n-butylamine with methanol in fixed bed reactor and results shown that the compound catalyst shows best performance when the mass fraction of alumina is 40%. It was found that the catalyst has good activity and selectivity for the N-methylation of aliphatic amines, but not for aromatic amines. For the aliphatic amines, the selectivity of second amines are better than primary amines because primary amine are prone to single methylation reaction to give N-methyl products. The low conversion of diisopropylamine indicated that the steric effect of amine also has a great influence for the N-methylation reaction over the compound catalyst. Besides, the test of the catalyst's stability was performed and the result demonstrated that the catalyst has constant stability and long lifetime. At last, the methanol adsorption was investigated by in situ FTIR in order to study the mechanism of dehydration reaction on the combined Al2O3–mordenite catalyst, and the methoxyl species which is the active species of the N-methylation of amines was found.Acknowledgements
The authors are grateful for the financial support from the Natural Science Foundation of China (21376213, 21476194), the Zhejiang Provincial Public Technology Research of China (2014C31123, 2015C31038) and the Low Carbon Fatty Amine Engineering Research Center of Zhejiang Province (2012E10033).References
- R. C. S. Araujo, V. M. D. Pasa and B. N. Melo, Eur. Polym. J., 2005, 41, 1420–1428 CrossRef CAS.
- M. Vijayaraj and C. S. Gopinath, Appl. Catal., A, 2007, 320, 64–68 CrossRef CAS.
- X. Ge, C. X. Luo, C. Qian, Z. P. Yu and X. Z. Chen, RSC Adv., 2014, 4, 43195–43203 RSC.
- A. Del Zotto, W. Baratta, M. Sandri, G. Verardo and P. Rigo, Eur. J. Inorg. Chem., 2004, 524–529 CrossRef CAS.
- M. V. Klyuev and M. L. Khidekel, Usp. Khim., 1980, 49, 28–53 CAS.
- A. Baiker and J. Kijenski, Catal. Rev., 1985, 27, 653–697 CAS.
- H. G. Chen, T. Zhang, M. Luo, C. Qiao, J. Q. Liu, X. Han and X. Z. Chen, J. Chem. Eng. Chin. Univ., 2009, 23, 801–806 CAS.
- Q. X. Li, G. Y. Zhang and S. Y. Peng, Chin. J. Catal., 2001, 22, 7–10 CAS.
- X. M. Liu, Fine Chemicals, 2002, 19, 315–317 CAS.
- X. Y. Mao and X. Z. Chen, J. Zhejiang Univ., Sci., B, 2003, 37, 307–309 CAS.
- J. M. Huang, C. Qian, F. Lie, Y. B. Chen and X. Z. Chen, Monatsh. Chem., 2013, 144, 1187–1190 CrossRef CAS.
- J. M. Huang, L. F. Xu, C. Qian and X. Z. Chen, Chem. Pap., 2012, 66, 304–307 CAS.
- X. Zhang, T. Zhang, X. X. Chen, H. Yan, M. Luo, C. Qian and X. Z. Chen, J. Zhejiang Univ., Sci., B, 2009, 43, 1684–1686 CAS.
- X. Ge, J. B. Pan, C. Qian, L. Feng, Y. B. Chen and X. Z. Chen, Catal. Commun., 2014, 46, 201–207 CrossRef CAS.
- X. Y. Hou, J. L. Qin and G. S. Zhou, China Surfactant Deterg. Cosmet., 2004, 34, 283–286 CAS.
- A. Baiker, D. Monti and Y. S. Fan, J. Catal., 1984, 88, 81–88 CrossRef CAS.
- S. Narayanan and K. Deshpande, Appl. Catal., A, 2000, 199, 1–31 CrossRef CAS.
- F. Valot, F. Fache, R. Jacquot, M. Spagnol and M. Lemaire, Tetrahedron Lett., 1999, 40, 3689–3692 CrossRef CAS.
- C. Grundling, G. EderMirth and J. A. Lercher, J. Catal., 1996, 160, 299–308 CrossRef.
- L. Abrams, M. Keane and G. C. Sonnichsen, J. Catal., 1989, 115, 410–419 CrossRef CAS.
- D. T. Chen, L. Zhang, C. Yi and J. A. Dumesic, J. Catal., 1994, 146, 257–267 CrossRef CAS.
- V. A. Veefkind and J. A. Lercher, Appl. Catal., A, 1999, 181, 245–255 CrossRef CAS.
- L. Kubelkova, J. Novakova and K. Nedomova, J. Catal., 1990, 124, 441–450 CrossRef CAS.
- L. W. Zhang, J. H. Wang, P. Wu, Z. P. Hou, J. H. Fei and X. M. Zheng, Chin. J. Catal., 2010, 31, 987–992 CrossRef CAS.
- S. Göbölös, M. Hegedüs, I. Kolosova, M. Maciejewski and J. L. Margitfalvi, Appl. Catal., A, 1998, 169, 201–206 CrossRef.
- I. I. Ivanova, E. B. Pomakhina, A. I. Rebrov, M. Hunger, Y. G. Kolyagin and J. Weitkamp, J. Catal., 2001, 203, 375–381 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |