Enhancement of yellow persistent luminescence in Eu2+-doped β-Ba3P4O13 phosphor by Ga3+ codoping
Wenbo Chen,
Yuhua Wang*,
Wei Zeng,
Gen Li and
Haijie Guo
Key Laboratory for Special Function Materials and Structural Design of the Ministry of the Education, School of Physical Science and Technology, Lanzhou University, Lanzhou, 730000, China. E-mail: wyh@lzu.edu.cn; Fax: +86-931-8913554; Tel: +86-931-8912772
First published on 11th May 2016
Abstract
Novel yellow emitting persistent phosphors β-Ba3P4O13:Eu2+ and β-Ba3P4O13:Eu2+,Ga3+ were successfully synthesized via solid-reaction method. Ga3+ was proved to be an effective codopant to enhance persistent luminescence in Eu2+-doped β-Ba3P4O13 phosphors. Eu2+–Ga3+ codoped β-Ba3P4O13 showed about 6 times longer persistence time than the Eu2+-singly-doped phosphors. The yellow long persistent luminescence (LPL) of β-Ba3P4O13:Eu2+,Ga3+ can persist about 10 h. The thermoluminescence (TL) analyses showed that co-doping Ga3+ can improve more appropriate traps leading to the enhancement of the afterglow. These results suggested that Ga3+ may play a critical role in creating traps in β-Ba3P4O13:Eu2+.
Introduction
Long persistent phosphors, exhibiting an emission of light lasting for hours after the excitation stoppage, have attracted considerable attention for their wide application in security signs, optical storage media, radiation detection and in vivo bio-imaging.1–4Eu2+ is the most widely used activator for persistent phosphors because its 5d electron state is usually close to the conduction band of the host, which makes trapping of electrons possible.5 Codoping trivalent rare earth ions has been a general way to improve the LPL of Eu2+ doped compounds since the afterglow properties of SrAl2O4:Eu2+,Dy3+ (ref. 6) were vastly improved via co-doping Dy3+ in SrAl2O4:Eu2+. By our observation, Dy3+ or Nd3+ are good candidates for Eu2+ active aluminates and silicates,7–11 while Tm3+ is often chosen for Eu2+active nitrides.12,13 However, for Eu2+-activated phosphate persistent phosphors, the above-mentioned rare earth ions usually have little influence on their persistent luminescence properties.14 More importantly, finding an appropriate codopant does not only benefit the apparent optical properties, but also contributes to revealing some important information on their mechanism. Hence, there is a strong desire for exploring suitable codopants to improve the LPL properties of Eu2+-activated phosphates.
Ba3P4O13 has two polymorphic forms with a temperature of transformation of 870° ± 10.15,16 The low-temperature form is triclinic and the high-temperature form is orthorhombic, labeled as α and β, respectively. In this paper, we report a novel LPL of Eu2+ doped β-Ba3P4O13. It is found that the incorporation of Ga3+ into β-Ba3P4O13:Eu2+ could remarkably enhance its LPL properties. The mechanism for the origin and enhancement of LPL is also discussed briefly.
Experimental
The investigated samples in this work were synthesized through the high temperature solid-state method with BaCO3 (A. R.), NH4H2PO4 (A. R.), Eu2O3 (99.99%) and Ga2O3 (99.99%) as raw materials. Starting materials were homogeneously mixed in an agate mortar by adding a known amount of ethanol, and ground for 1 h. The mixture was then placed into an alumina crucible and sintered at 1173 K for 2 h under reducing atmosphere in an electric tube furnace. Finally, after calcination, the samples were cooled to room temperature in the furnace and ground again into powder for subsequent use.All the phase structures of samples were characterized by powder X-ray diffraction using a Rigaku diffractometer with Ni-filtered Cu Kα radiation at a scanning step of 0.02° in the 2θ range from 10° to 80°. Excitation and emission spectra were obtained by a FLS-920T fluorescence spectrophotometer with Xe 900 (450 W xenon arc lamp) as the light source. The afterglow decay curves were recorded using a PR305 long afterglow instrument after the samples were irradiated by ultraviolet light (254 nm) for 10 min. The thermoluminescence curves were measured by a FJ-427A1 meter (Beijing Nuclear Instrument Factory) with a heating rate of 1 K s−1. Before the measurement, the samples were irradiated by ultraviolet light (254 nm) for different time. All the data were measured at room temperature except for the thermoluminescence curves.
Results and discussion
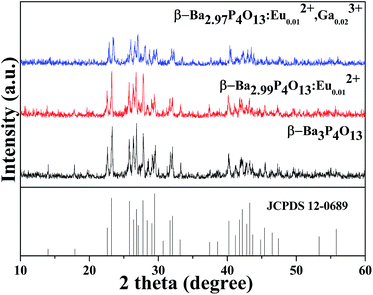
In this work, the effect of Eu2+ and Ga3+ doping concentration on LPL properties are investigated. The optimum phosphor is β-Ba2.97P4O13:Eu0.012+,Ga0.023+ in which the persistent time achieves maximum. The optimum concentration is 0.01 mol in case of Eu2+ single doped β-Ba3P4O13. Only the optimum phosphors β-Ba2.99P4O13:Eu0.012+ and β-Ba2.97P4O13:Eu0.012+,Ga0.023+ are discussed in this paper.Fig. 1 shows the typical X-ray diffraction (XRD) patterns of β-Ba3P4O13, β-Ba2.99P4O13:Eu0.012+ and β-Ba2.97P4O13:Eu0.012+,Ga0.023+. The results reveal that a predominant phase of β-Ba3P4O13 (JCPDS no. 12-0689) is presented in all powder samples. No phase transformation or impurity is observed, clearly implying that the doping Eu2+ and Ga3+ does not cause any significant change in host structure.
The PLE and PL spectra of β-Ba2.99P4O13:Eu0.012+ and β-Ba2.97P4O13:Eu0.012+,Ga0.023+ are depicted in Fig. 2a. The PLE spectrum shows a strong broad band from 250 nm to 450 nm, which is attributed to the 4f7 → 4f65d1 transition of the Eu2+ ions. The PL of the β-Ba2.97P4O13:Eu0.012+,Ga0.023+ phosphor shows broad asymmetric emission band from 400 nm to 800 nm, which is attributed to the 4f65d1 → 4f7 transition of the Eu2+ ions. The spectral shapes are almost identical in the two samples. With regard to the relative intensity, β-Ba2.97P4O13:Eu0.012+,Ga0.023+ shows about 4 times higher intensity than the β-Ba2.99P4O13:Eu0.012+ in the PL and PLE spectra. The improvement of photoluminescence efficiency by Ga3+ codopants can be associated with size mismatches between the Ga3+ and the host cation.17–19 The charge mismatch resulted in the creation of the charge compensating Ba vacancy. The Ba vacancy situated around the Eu2+ lowered the local site-symmetry of the Eu2+. This possibly gave rise to a stronger effect on the enhancement of the luminescence. By Gaussian deconvolution (Fig. 2b), the emission spectra β-Ba3P4O13:Eu2+,Ga3+ phosphor can be decomposed into two Gaussian profiles with peaks centered at 558 nm (2.22 eV) and 610 nm (2.03 eV), respectively. Based on the consideration of effective ionic radii with different coordination numbers, the doping rare-earth ions, Eu2+ are proposed to occupy the regular Ba2+ sites rather than P5+ sites in the β-Ba3P4O13 host lattice. The two peaks can be identified as the different emission environments of the Ba2+ ions being occupied by Eu2+ ions.
 | ||
| Fig. 2 (a) PLE and PL spectra of β-Ba2.99P4O13:Eu0.012+ and β-Ba2.97P4O13:Eu0.012+,Ga0.023+. (b) The deconvoluted Gaussian components of Ba2.97P4O13:Eu0.012+,Ga0.023+. | ||
The LPL decay curve of β-Ba2.99P4O13:Eu0.012+ and β-Ba2.97P4O13:Eu0.012+,Ga0.023+ are presented in Fig. 3a. It can be seen that the persistent time of β-Ba2.99P4O13:Eu0.012+ is only 1.5 h. An encouraging result of the present work is that by co-doping Ga3+, the persistent time increases largely. The persistent time of the β-Ba2.97P4O13:Eu0.012+,Ga0.023+ is nearly 10 h at a recognizable intensity level (≧0.32 mcd m−2). The decay curves and fitting curves of β-Ba2.97P4O13:Eu0.012+,Ga0.023+ phosphor are presented inset in Fig. 4a. It is clearly exhibits that the decay process consists of a fast decay process and a slow decay part which are well-fitted into a biexponential function as follows:
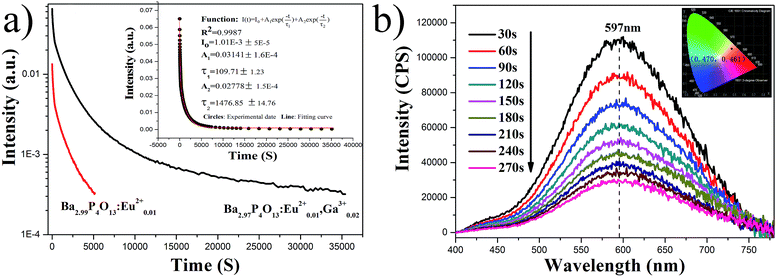 | ||
| Fig. 3 (a) LPL decay curve of two samples. (b) Persistent luminescence spectra of Ba3P4O13:Eu2+,Ga3+ and corresponding CIE chromaticity diagram. | ||
 | ||
| Fig. 4 TL curves (a) and deconvolution of TL glow curve (b and c) of Ba2.99P4O13:Eu0.012+ and Ba2.97P4O13:Eu0.012+,Ga0.023+. | ||
| Sample | A1 | A2 | τ1 | τ2 |
|---|---|---|---|---|
| Ba2.99P4O13:Eu0.012+ | 0.0073 | 0.0049 | 91 | 981 |
| Ba2.97P4O13:Eu0.012+,Ga0.023+ | 0.031 | 0.027 | 110 | 1477 |
The significant role of traps has been recognized in the field of LPL and the persistent luminescence is governed by the slow liberation of trapped charge carriers by thermal stimulation.20–22
In order to characterize the traps, TL measurements are performed on β-Ba2.99P4O13:Eu0.012+ and Ga3+ codoped sample β-Ba2.97P4O13:Eu0.012+,Ga0.023+ and their TL curves are illustrated in Fig. 4a. It is obvious that the shape of TL curve of Ga3+ sample is very similar to that of Eu2+ single doped sample, which indicated that the codopant Ga3+ does not import new trap into the phosphor. Compared with Eu2+ single doped sample, the TL intensity of Ga3+ codoped sample increases largely, suggesting the concentration of trap increases by codoping Ga3+. The deconvolution of TL glow curves of β-Ba2.97P4O13:Eu0.012+,Ga0.023+ and β-Ba2.99P4O13:Eu0.012+ are shown in Fig. 4b and c, respectively. It is obvious that there are two traps in both single doped and co-doped samples. By codoping Ga3+ in the sample, the intensity of T1 is increased largely, whereas the T2 is nearly invariable. As indicated above, the persistent time of co-doped sample is prolonged nearly six times compared with that of the Eu2+ single-doped sample. Therefore, the T1 may be responsible for the yellow LPL. In order to further verify assumption, the trap densities (n0) and trap depths (Et) are calculated by using Chen's equation.23,24
| Et = [2.52 + 10.2 × (μg − 0.42)] × (kBTm2)/ω − 2kBTm |
| n0 = ωIm/{β × [2.52 + 10.2 × (μg − 0.42)]} |
 is easy to form.5,25 With the codoping of Ga3+, the lattice distortion is further strengthened because the ion radius of Ba2+ and Ga3+ is much difference. Hence, more
is easy to form.5,25 With the codoping of Ga3+, the lattice distortion is further strengthened because the ion radius of Ba2+ and Ga3+ is much difference. Hence, more  could be generated, resulting in the enhancement of TL band. Thus, the TL band can be attributed to the generation of positive charge defect
could be generated, resulting in the enhancement of TL band. Thus, the TL band can be attributed to the generation of positive charge defect  which can serve as electron traps in Eu2+ single-doped and Ga3+-codoped samples. Under UV excitation, electrons are promoted from the occupied 4f levels of Eu2+ to conduction band. The excited electrons are subsequently captured by
which can serve as electron traps in Eu2+ single-doped and Ga3+-codoped samples. Under UV excitation, electrons are promoted from the occupied 4f levels of Eu2+ to conduction band. The excited electrons are subsequently captured by 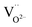 through the conduction band. Trapped electrons can exist for a long time at room temperature even after the irradiation light source is removed. Under thermal agitation, stored electrons can be released back to the conduction band, then electrons transfer to the 5d levels of Eu2+, which leads to the 4f65d1–4f7 (8S7/2) yellow LPL.
through the conduction band. Trapped electrons can exist for a long time at room temperature even after the irradiation light source is removed. Under thermal agitation, stored electrons can be released back to the conduction band, then electrons transfer to the 5d levels of Eu2+, which leads to the 4f65d1–4f7 (8S7/2) yellow LPL.
| Sample | Tm (K) | Et (eV) | n0 (cm−3) |
|---|---|---|---|
| Eu2+,Ga3+ | 335 | 0.78 | 1.38 × 107 |
| Eu2+ | 332 | 0.76 | 7.20 × 106 |
Conclusion
A new yellow long-lasting phosphor β-Ba3P4O13:Eu2+,Ga3+ is synthesized by a solid state reaction. The Eu2+ substituting the Ba sites show an asymmetric emission band centered at 597 nm. The persistent time of β-Ba2.99P4O13:Eu0.012+ is only 1.5 h. Codoping non-rare earth Ga3+ can increase the intensity T1, resulting in the improvement of afterglow properties. The duration of Eu2+,Ga3+ co-doped β-Ba3P4O13 is about 10 h at a recognizable intensity level (≧0.32 mcd m−2). This investigation provides a new and efficient non-rare codopant for Eu2+-activated phosphate persistent phosphors, which contribute to the mechanism of codopants.Acknowledgements
This work was supported by Specialized Research Fund for the Doctoral Program of Higher Education (No. 20120211130003) 35 and the National Natural Science Funds of China (Grant No. 51372105).Notes and references
- F. Liu, W. Yan, Y. J. Chuang, Z. Zhen, J. Xie and Z. Pan, Sci. Rep., 2013, 3, 1554 Search PubMed.
- T. Maldiney, A. Bessière, J. Seguin, E. Teston, S. K. Sharma, B. Viana, A. J. Bos, P. Dorenbos, M. Bessodes and D. Gourier, Nat. Mater., 2014, 13, 418–426 CrossRef CAS PubMed.
- J. Wang, H. Zhang, B. Lei, Z. Xia, H. Dong, Y. Liu, M. Zheng and Y. Xiao, J. Mater. Chem. C, 2015, 3, 4445–4451 RSC.
- P. Wang, X. Xu, D. Zhou, X. Yu and J. Qiu, Inorg. Chem., 2015, 54, 1690–1697 CrossRef CAS PubMed.
- F. Clabau, X. Rocquefelte, T. Le Mercier, P. Deniard, S. Jobic and M. H. Whangbo, Chem. Mater., 2006, 18, 3212–3220 CrossRef CAS.
- T. Matsuzawa, Y. Aoki, N. Takeuchi and Y. Murayama, J. Electrochem. Soc., 1996, 143, 2670–2673 CrossRef CAS.
- H. Yamamoto and T. Matsuzawa, J. Lumin., 1997, 72, 287–289 CrossRef.
- B. Cheng, L. Fang, Z. Zhang, Y. Xiao and S. Lei, J. Phys. Chem. C, 2011, 115, 1708–1713 CAS.
- Y. Lin, Z. Tang, Z. Zhang and C. W. Nan, Appl. Phys. Lett., 2002, 81, 996–998 CrossRef CAS.
- Z. Qiu, Y. Zhou, M. Lü, A. Zhang and Q. Ma, Acta Mater., 2007, 55, 2615–2620 CrossRef CAS.
- S. Cheng, X. Xu, J. Han, J. Qiu and B. Zhang, Powder Technol., 2015, 276, 129–133 CrossRef CAS.
- B. Lei, K.-i. Machida, T. Horikawa, H. Hanzawa, N. Kijima, Y. Shimomura and H. Yamamoto, J. Electrochem. Soc., 2010, 157, J196–J201 CrossRef CAS.
- J. Li, B. Lei, J. Qin, Y. Liu and X. Liu, J. Am. Ceram. Soc., 2013, 96, 873–878 CrossRef CAS.
- G. Ju, Y. Hu, L. Chen, X. Wang and Z. Mu, Opt. Mater., 2014, 36, 1920–1923 CrossRef CAS.
- J. Millet, H. Parker and R. Roth, J. Am. Ceram. Soc., 1986, 69, C-103–C-105 CrossRef.
- H. Guo, W. Chen, W. Zeng, Y. Wang and Y. Li, ECS Solid State Lett., 2015, 4, R1–R3 CrossRef CAS.
- E. Antic-Fidancev, J. Hölsä, M. Lastusaari and A. Lupei, Phys. Rev. B, 2001, 64, 195108 CrossRef.
- S.-I. Oh, Y.-K. Jeong and J.-G. Kang, Bull. Korean Chem. Soc., 2009, 30, 419–422 CrossRef CAS.
- X. Liu, K. Han, M. Gu, L. Xiao, C. Ni, S. Huang and B. Liu, Solid State Commun., 2007, 142, 680–684 CrossRef CAS.
- M. Allix, S. Chenu, E. Véron, T. Poumeyrol, E. A. Kouadri-Boudjelthia, S. Alahraché, F. Porcher, D. Massiot and F. Fayon, Chem. Mater., 2013, 25, 1600–1606 CrossRef CAS.
- T. Wang, W. Bian, D. Zhou, J. Qiu, X. Xu and X. Yu, J. Phys. Chem. C, 2015, 119, 14047–14055 CAS.
- T. Wang, J. Gou, X. Xu, D. Zhou, J. Qiu and X. Yu, Opt. Express, 2015, 23, 12595–12604 CrossRef PubMed.
- R. Chen, J. Appl. Phys., 1969, 40, 570–585 CrossRef CAS.
- R. Chen, J. Electrochem. Soc., 1969, 116, 1254–1257 CrossRef.
- X. Xu, Y. Wang, Y. Gong, W. Zeng and Y. Li, Opt. Express, 2010, 18, 16989–16994 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |