Urinary UPLC-MS metabolomics dissecting the underlying mechanisms of Huaxian capsule protects against sepsis†
Qun Liang*a,
Han Liub,
Haitao Xinga,
Yan Jianga and
Ai-Hua Zhang*a
aICU Center, First Affiliated Hospital, School of Pharmacy, Heilongjiang University of Chinese Medicine, Heping Road 24, Xiangfang District, Harbin 150040, China. E-mail: qunliang1970@163.com; zhanghuaomics2@163.com; Fax: +86-451-86053141; Tel: +86-451-86053141
bSimon Fraser University (SFU), Burnaby, British Columbia, Canada
First published on 18th April 2016
Abstract
Severe sepsis (SS) remains a worldwide threat, not only in industrialized countries but also in developing countries. Currently, the incidence of SS is rising, and there is a lack of effective drugs. Huaxian capsule (HXC) is traditional Chinese medicine with therapeutic effects on SS. The objectives of this study were to explore the underlying mechanisms of pharmacological effects of HXC on the treatment of SS. Metabolomics is a powerful tool for the characterization of metabolic phenotypes, potential metabolite biomarkers and metabolic pathways. Here, we investigated the metabolic changes in SS rats by performing metabolic profiling and metabolite biomarkers. Urinary UPLC-MS coupled with multivariate statistical analysis including principal components analysis (PCA), partial least-squares discriminant analysis (PLS-DA), and orthogonal projections to latent structures discriminant analysis (OPLS-DA) were used to assess phenotypic changes. Metabolites contributing to the discrimination the control group, SS group and HXC group were identified by using unsupervised PCA. Metabolic pathway analysis was performed using the MetPA web tool. As a result, 10 potential metabolite biomarkers in urine involved phenylalanine, tyrosine and tryptophan biosynthesis, valine, leucine and isoleucine biosynthesis, glycine, serine and threonine metabolism, tyrosine metabolism pathways, were identified in SS rats. The unsupervised PCA score plot showed that the HXC group cluster modulating dynamic metabolic changes was closer to that of the control group than the model group cluster, demonstrating that HXC has therapeutic effects on SS rats. Overall, it showed that urinary UPLC-MS metabolomics could dissect the underlying mechanisms of HXC protects against SS.
1. Introduction
Mortality and morbidity in severe sepsis (SS) remain high despite significant advances in critical care.1–4 Despite recent advances in antibiotic therapies, and there is an urgent need for biomarkers for therapeutically evaluation of the SS. TCM has been used as a regular treatment for diseases in Asian countries, because of its therapeutic effects of combination formulae.5,6 Under the guidance of the TCM medical theory, Huaxian capsule (HXC) is a TCM prescription with a long history used in the clinic for the treatment of SS. However, little work has been conducted on its metabolic profile and metabolic biomarkers. A detailed understanding of its metabolic processes will likely provide much needed clinical insights.The action mechanism characterization of metabolic phenotypes, potential metabolite biomarkers and metabolic pathways is unclear.
Metabolomics is an high-throughput bioanalytical technology aiming to identify and quantify small molecules present in any biological system.7 Metabolomics can dynamically operate via high-throughput detection and data processing to obtain potential biomarkers, which is conducive to the study of TCM.8–14 It could be used to identify significantly different metabolites in the physiological state of a biological system. Metabolic profiling can provide a new opportunity to explore the global metabolic effects of TCM. Some studies indicate that urinary metabolic profiles are promising host biomarkers for the detection of potential biomarkers changes and could contribute to early treatment and prevention of diseases.15–19 Therefore, to explore the underlying mechanisms of the therapeutic effects of HXC in a model of SS, present study with metabolomics was performed to discover potential metabolites by analysis of urine samples. To explore potential characteristic metabolites signatures associated with SS, this study will help researchers better understand the underlying metabolic mechanisms of HXC in the treatment of SS.
2. Materials and methods
2.1 Drugs and reagents
Acetonitrile and methanol (HPLC grade) were purchased from Fisher Inc., UK. Water was produced by a Milli-Q Ultra-pure water system (Millipore, USA); formic acid was obtained from Honeywell Co. Ltd, USA; leucine enkephalin was provided by Sigma-Aldrich Co. Ltd, USA. All other reagents were HPLC grade. Huaxian capsule (HXC) was a clinical preparation and provided by First Affiliated Hospital of Heilongjiang University of Chinese Medicine (Harbin, China).2.2 Experimental animal
The protocol was approved by the Committee on the Ethics of Animal Experiments of Heilongjiang University of Chinese Medicine. Adult male Wistar rats (220 ± 20 g) of specified pathogen free (SPF) were purchased from SLAC Laboratory Animal Co. (Shanghai, China). All rats were placed in standard cages under standard temperature conditions at 24 ± 2 °C with 40 ± 5% humidity and a 12 h light/dark cycle.2.3 Sepsis model
The cecal ligation and puncture (CLP) method was used as a SS model, as described in the literature.20 Under ether anesthesia, a midline incision was made and the cecum was divided carefully. The distal two thirds of the cecum was punctured through twice with an 18-gauge needle and ligated tightly. And then, the cecum was placed back in the peritoneal cavity and the abdominal cavity was closed. In sham surgical controls, the cecum was exposed but not punctured and ligated before being returned to the abdominal cavity. The animals were then allowed to acclimatize and putted in the metabolism cages.2.4 Sample collection and preparation
The rats were randomly divided into model group (n = 8, treated with 0.2 mL solution immediately and 24 h after CLP), HXC treatment group (n = 8, orally administered at a dose of 2 g kg−1 of body weight once a day and 24 h after CLP), and sham (control) group (n = 8, treated with shame operation and the same volume of 0.9% saline solution). Drug or vehicle was administrated between 8:00 and 9:00 am to minimize any effects of the circadian rhythm. After 3 consecutive days of drug administration, all of the animals were sacrificed by exsanguination from the abdominal aorta under isoflurane anesthesia. The urine was collected and centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C to remove any solid debris, and then stored at −80 °C for analysis. Thawed urine samples were collected after centrifugation at 13
000 rpm for 10 min at 4 °C to remove any solid debris, and then stored at −80 °C for analysis. Thawed urine samples were collected after centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C, and the supernatant was transferred to a auto-sampler injection vial, and then filtered through a syringe filter (0.22 μm), 5 μL of the supernatant were injected into the UPLC/MS. To ensure the analytical variability and to monitor the data acquisition performance in the whole run, the pooled quality control (QC) samples were prepared from each sample and staggered with the other samples.
000 rpm for 10 min at 4 °C, and the supernatant was transferred to a auto-sampler injection vial, and then filtered through a syringe filter (0.22 μm), 5 μL of the supernatant were injected into the UPLC/MS. To ensure the analytical variability and to monitor the data acquisition performance in the whole run, the pooled quality control (QC) samples were prepared from each sample and staggered with the other samples.
2.5 UPLC-QTOF/MS analysis
Metabolome analysis of urine samples by UPLC-ESI-TOF/MS (Waters Corporation, Milford, MA). An Acquity UPLC BEH-C18 column (2.1 mm × 100 mm × 1.8 μm) which was maintained at 40 °C and was used for all analyses. A gradient of 0.1% formic acid in water (solvent A) and acetonitrile (solvent B) was used. The gradient used for urine samples was as follows: 0–5 min, 1–25% B; 5–8 min, 25–50% B; 8–9 min, 50–99% B; 9–10 min, 99% B; 10–11 min, 99–1% B; 11–12 min, 1% B. The flow rate was set to 0.2 mL min−1, and a 5 μL aliquot of the sample solution was injected into the system. All the samples were maintained at 4 °C during the analysis. Mass spectrometry was performed on a Q-TOF mass spectrometer (Waters, Manchester, U. K.), and the parameters were set as follows: capillary voltage, 2.5 kV; source temperature, 120 °C; desolvation temperature, 550 °C; cone gas flow, 50 L h−1; and desolvation gas flow, 500 L h−1. Centroid data were collected from 50 to 1500 m/z with a scan time of 0.03 s, and an inter scan delay of 0.02 s. Leucine-enkephalin was used as the lock mass (m/z 556.2771 in ESI+ and 554.2615 in ESI−).2.6 Data analysis
All the data was processed using MassLynx V4.1 software (Waters Corp., Milford, MA). The mass data acquired were analyzed using EZinfo 2.0 software (Waters Corp, Milford, USA) for peak detection and alignment. EZinfo 2.0 software was then used to perform unsupervised principal components analysis (PCA) and partial least squares discriminant analysis (PLS-DA) and orthogonal partial least-squared discriminate analysis (OPLS-DA). PCA was used to reveal the global metabolic changes. PLS-DA and OPLS-DA were applied to determine the various metabolites responsible for the separation between the model and sham-operated groups. The corresponding variable importance in the projection (VIP value) was calculated in the OPLS-DA model was used to identify metabolites contributing to the discrimination. The normalized data were then uploaded to MetaboAnalyst 3.0 (ref. 21) for hierarchical clustering analysis. Hierarchical clustering was performed and heat maps were created based on the Pearson distance measure and the Ward clustering algorithm.2.7 Metabolites identifications and metabolic annotations
Metabolites were identified using MassLynx V4.1 software (Waters Corp., Milford, MA). For the identification of potential biomarkers, some available biochemical databases, the online database Chemspider (http://www.chemspider.com), HMDB (http://www.hmdb.ca/), KEGG (http://www.genome.jp/kegg/), and SMPD (http://www.smpdb.ca/) were used to annotate the metabolites by matching the exact molecular mass data (m/z) with those from the database. If a mass difference between observed and the database value was less than 5 ppm, the metabolite would be annotated and the molecular formula of metabolites would further be identified and validated by the isotopic distribution measurements. Reference standards were purchased and used to validate and confirm those significantly changed metabolites by comparing their MS/MS spectra and retention time. Pathway analysis of those significantly changed metabolites was performed with MetPA Pathway Analysis (http://metpa.metabolomics.ca/MetPA), based on KEGG, and SMPD database and facilitate further biological interpretation.3. Results and discussion
3.1 Total ion chromatogram of urine samples
Untargeted metabolomics analysis was performed for urine samples. The UPLC-ESI-TOF/MS TIC chromatograms of urine samples from the model group, HXC treated group, and sham (control) group are shown in Fig. S1.† In order to illustrate the differences of the metabolic profiles, UPLC-ESI-TOF/MS spectra were further pretreated and a pattern recognition analysis was carried out.3.2 Metabolic findings in urine samples
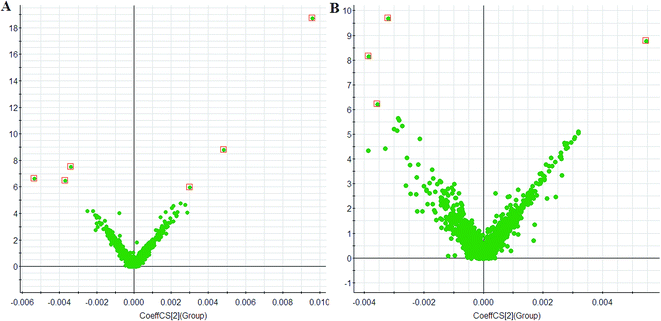
The metabolic profiles of urine changed significantly patterns on 3 day. The PCA scores plot showed distinct clustering between the control and model group (Fig. 1), and indicate that metabolites have been significantly perturbed in the model group. This shows that the urinary metabolic profiles can indeed be useful for discriminating between model group, and sham (control) group. The potentially interesting metabolites biomarkers were identified and selected by using the cutoff of PLS-DA Variable Importance in Projection (VIP) score (Fig. 2). Using the criteria that VIP was larger than 6, ten endogenous metabolites were selected as biomarkers.22 Following the identification process, these metabolites were identified over a narrow ±5 ppm, including 6 identified in the positive mode and 4 identified in the negative mode (Table 1). Intriguingly, 6 of the metabolites detected were found to be up-regulated, while 4 were down-regulated in the model group compared to the control group.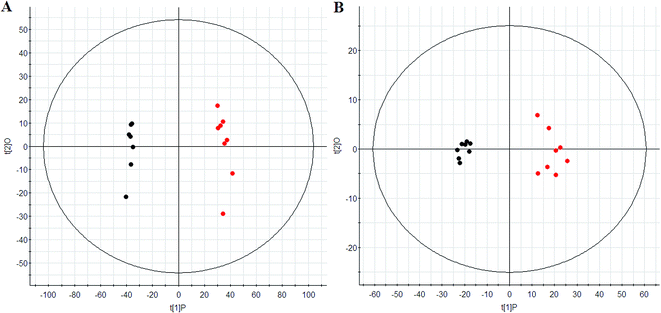 | ||
| Fig. 1 PCA score plots of urine of control (red) and model group (black) in ESI+ (A) and ESI− (B) mode. | ||
| No. | VIP | Retention time (min) | m/z | Adducts | Formula | Mass error (ppm) | Description | Anova (p) | Trend |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.48 | 1.21 | 138.06 | M + H | C7H7NO2 | −1.90 | 2-Aminobenzoic acid | 0.0013 | Down |
| 2 | 5.98 | 1.54 | 132.10 | M + H | C6H13NO2 | 0.24 | L-Leucine | 0.0053 | Up |
| 3 | 7.54 | 2.72 | 180.09 | M + H | C6H13NO5 | 0.26 | Glucosamine | 0.0446 | Up |
| 4 | 18.71 | 3.48 | 188.07 | M + H | C11H9NO2 | −4.23 | Indoleacrylic acid | 0.0007 | Down |
| 5 | 6.64 | 3.81 | 190.05 | M + H | C10H7NO3 | −2.80 | Kynurenic acid | 0.0422 | Up |
| 6 | 8.79 | 3.98 | 182.08 | M + H | C9H11NO3 | −1.25 | L-Tyrosine | 0.0258 | Down |
| 7 | 9.69 | 1.36 | 178.07 | M − H | C9H9NO3 | −2.62 | Hippuric acid | 0.0273 | Up |
| 8 | 8.15 | 2.65 | 74.04 | M − H | C2H5NO2 | −1.08 | Glycine | 0.0088 | Up |
| 9 | 6.23 | 4.25 | 190.07 | M − H | C10H9NO3 | 0.44 | 5-Hydroxyindoleacetic acid | 0.0176 | Up |
| 10 | 8.79 | 6.87 | 196.11 | M − H | C10H15NO3 | −3.99 | Metanephrine | 0.0075 | Down |
3.3 Metabolic pathway analysis
Metabolites selected by filtering the dataset using the cutoff of PLS-DA VIP score >6 between the different groups were analyzed using MetPA, and mapped onto likely relevant pathways, and were used to explain the metabolism. This analysis resulted in the construction of 16 metabolic pathways in the plasma (Fig. 3 and Table S1†) that were important for host-responses to SS. In addition, the metabolism of the phenylalanine, tyrosine and tryptophan biosynthesis (impact-value: 0.50), valine, leucine and isoleucine biosynthesis (impact-value: 0.33), glycine, serine and threonine metabolism (impact-value: 0.29), tyrosine metabolism (impact-value: 0.14) in urine were determined to be the most important. Metabolic pathways associated with SS had significantly altered metabolism compared to the normal state.3.4 Metabolic effects of HXC treatment
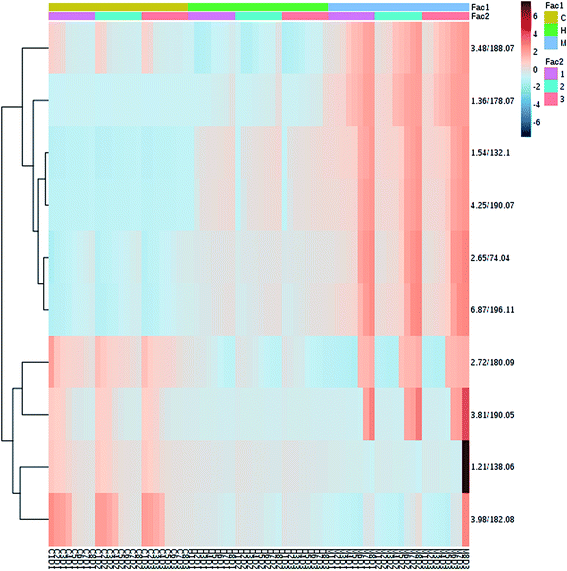
MS data of urine samples collected at different days were analyzed. The PCA score plot was used to investigate the metabolic effects of HXC intervention. As shown in Fig. 4 and 5, the clusters representing the HXC treatment group was located between the clusters representing the model and control groups, which demonstrated that the disturbed metabolic states of model group was in the process of returning to the normal state after HXC treatment. Compared to the model group, HXC treated group showed the recovery performance from the model metabolic state. The score plot showed that the HXC-treated group cluster was closer to that of the control groups than the model group cluster, which demonstrates that HXC has therapeutic effects on SS rats (Fig. 4). To better visualize the changes in the potential biomarkers, Fig. 5 shows a heat map of metabolites varied significantly among these groups using hierarchical clustering of time series analysis carried out by MetaboAnalyst 3.0, which indicate that the effectiveness of HXC as an SS treatment depends on modulating the potential biomarkers. | ||
| Fig. 4 PCA scores plot of HXC affecting on SS rats in ESI+ (A) and ESI− (B) mode. Note: control group (red), model group, HXC group (black). | ||
4. Conclusions
In conclusion, to our knowledge, this is the first study to identify a group of urinary metabolites signatures associated with SS using a UPLC/MS-based metabolomic approach. In this work, ten potential marker metabolites were identified. These SS-related metabolites regulated the phenylalanine, tyrosine and tryptophan biosynthesis, valine, leucine and isoleucine biosynthesis, glycine, serine and threonine metabolism, tyrosine metabolism. Metabolic profiling analysis verified the therapeutic effect of HXC. This study would aid into the elucidation of the therapeutic mechanism of HXC. It should be performed to assess their predictive value for the early diagnosis of these metabolites in clinic, and further studies with a larger sample size are needed to investigate any associations between metabolites and disease.Conflict of interest
The authors declare no competing financial interests.Acknowledgements
We thank BGI for excellent technical assistance. This work was supported by grants from the Key Program of Natural Science Foundation of State (Grant No. 81470196, 81302905), Natural Science Foundation of Heilongjiang Province of China (H2015038), Youth Innovative Talent Program of Heilongjiang Province of China (UNPYSCT-2015118).References
- X. Shi, F. Yang, Y. N. Zheng, H. Zhang, X. X. Wang, G. J. Shao and X. L. Lai, Metabolomic approach for the identification of therapeutic targets of erythropoietin against sepsis in rat models, Eur. Rev. Med. Pharmacol. Sci., 2016, 20(3), 537–546 CAS.
- Q. Liang, H. Liu, T. Zhang, Y. Jiang, H. Xing and A. Zhang, High-throughput metabolic profiling for discovering metabolic biomarkers of sepsis-induced acute lung injury, RSC Adv., 2016, 6, 11008–11013 RSC.
- M. Garcia-Simon, J. M. Morales, V. Modesto-Alapont, V. Gonzalez-Marrachelli, R. Vento-Rehues, A. Jorda-Miñana, J. Blanquer-Olivas and D. Monleon, Prognosis Biomarkers of Severe Sepsis and Septic Shock by 1H NMR Urine Metabolomics in the Intensive Care Unit, PLoS One, 2015, 10(11), e0140993 Search PubMed.
- Q. Liang, H. Liu, T. Zhang, Y. Jiang, H. Xing and A. Zhang, Potential urine biomarkers from a high throughput metabolomics study of severe sepsis in a large Asian cohort, RSC Adv., 2015, 5, 102204–102209 RSC.
- A. H. Zhang, H. Sun, G. L. Yan, P. Wang, Y. Han and X. J. Wang, Chinmedomics: a new strategy for research of traditional Chinese medicine, Zhongguo Zhongyao Zazhi, 2015, 40(4), 569–576 Search PubMed.
- J. Chen, J. Zhou, S. Wei, Z. Xie, C. Wen and G. Xu, Effect of a traditional Chinese medicine prescription Quzhuotongbi decoction on hyperuricemia model rats studied by using serum metabolomics based on gas chromatography-mass spectrometry, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2015, S1570–0232(15), 30250–30256 Search PubMed.
- A. H. Zhang, H. Sun, Y. Han, G. L. Yan, Y. Yuan, G. C. Song, X. X. Yuan, N. Xie and X. J. Wang, Ultra performance liquid chromatography-mass spectrometry based comprehensive metabolomics combined with pattern recognition and network analysis methods for characterization of metabolites and metabolic pathways from biological data sets, Anal. Chem., 2013, 85(15), 7606–7612 CrossRef CAS PubMed.
- Y. Nan, X. Zhou, Q. Liu, A. Zhang, Y. Guan, S. Lin, L. Kong, Y. Han and X. Wang, Serum metabolomics strategy for understanding pharmacological effects of ShenQi pill acting on kidney yang deficiency syndrome, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2015, S1570–0232(15), 30328–30337 Search PubMed.
- H. Chu, H. Sun, G. Yan, A. Zhang, C. Liu, H. Dong, X. Meng and X. Wang, Metabolomics analysis of health functions of Physalis pubescens L. using by ultra-performance liquid chromatography/electrospray ionization quadruple time-of-flight mass spectrometry, J. Tradit. Chin. Med., 2015, 1(3), 1–12 CrossRef.
- A. Zhang, H. Sun, S. Dou, W. Sun, X. Wu, P. Wang and X. Wang, Metabolomics study on the hepatoprotective effect of scoparone using ultra-performance liquid chromatography/electrospray ionization quadruple time-of-flight mass spectrometry, Analyst, 2013, 138(1), 353–361 RSC.
- H. Cao, A. Zhang, H. Zhang, H. Sun and X. Wang, The application of metabolomics in traditional Chinese medicine opens up a dialogue between Chinese and Western medicine, Phytother. Res., 2015, 29(2), 159–166 CrossRef PubMed.
- Q. Liang, C. Wang, H. Wu, Y. Zhu and A. Zhang, Metabolite fingerprint analysis of cervical cancer using LC-QTOF/MS and multivariate data analysis, Anal. Methods, 2014, 6, 3937 RSC.
- C. Liu, A. Zhang, Y. Han, S. Lu, H. Sun, G. Yan, P. Wang and X. Wang, Ultra-high performance liquid chromatography coupled with time-of-flight mass spectrometry screening and analysis of potential bioactive compounds from traditional Chinese medicine Kai-Xin-San, using a multivariate data processing approach and the MetaboLynx tool, RSC Adv., 2015, 5, 85–92 RSC.
- H. Chu, A. Zhang, Y. Han and X. Wang, Metabolomics and its potential in drug discovery and development from TCM, J. Tradit. Chin. Med., 2015, 1(4), 26–32 CrossRef.
- H. Jiang, J. Liu, T. Wang, J. R. Gao, Y. Sun, C. B. Huang, M. Meng and X. J. Qin, Mechanism of Xinfeng Capsule on Adjuvant-Induced Arthritis via Analysis of Urinary Metabolomic Profiles, Autoimmune Dis., 2016, 2016, 5690935 Search PubMed.
- Q. Liang, H. Liu, T. Zhang, Y. Jiang, H. Xing and A. Zhang, High-throughput metabolic profiling for discovering metabolic biomarkers of sepsis-induced acute lung injury, RSC Adv., 2016, 6, 11008–11013 RSC.
- Q. Liang, H. Liu, T. Zhang, Y. Jiang, H. Xing and A. Zhang, Discovery of serum metabolites for diagnosis of mild cognitive impairment to Alzheimer's disease progression using an optimized metabolomics method, RSC Adv., 2016, 6, 3586–3591 RSC.
- Q. Liang, H. Liu, T. Zhang, Y. Jiang, H. Xing and A. Zhang, Potential urine biomarkers from a high throughput metabolomics study of severe sepsis in a large Asian cohort, RSC Adv., 2015, 5, 102204–102209 RSC.
- Q. Liang, T. Zhang, Y. Jiang, H. Xing, C. Wang, B. Li and A. Zhang, Metabolomics of Alcoholic Liver Disease: A Clinical Discovery Study, RSC Adv., 2015, 5, 80381–80387 RSC.
- K. A. Wichterman, A. E. Baue and I. H. Chaudry, Sepsis and septic shock–a review of laboratory models and a proposal, J. Surg. Res., 1980, 29(2), 189–201 CrossRef CAS PubMed.
- J. Xia, I. V. Sinelnikov, B. Han and D. S. Wishart, MetaboAnalyst 3.0–making metabolomics more meaningful, Nucleic Acids Res., 2015, 43(W1), W251–W257 CrossRef PubMed.
- Y. Li, S. Qiu and A. Zhang, High-throughput metabolomics to identify metabolites serve as diagnostic biomarkers of prostate cancer, Anal. Methods, 2016 10.1039/C6AY00127K.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra07987c |
| This journal is © The Royal Society of Chemistry 2016 |