Influence of the D/A ratio of 1,3,5-triphenylbenzene based starburst host materials on blue electrophosphorescent devices: a comparative study
Abstract
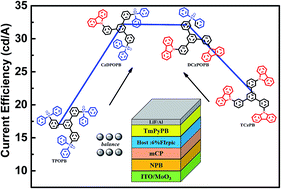
A series of starburst host materials, composed of a central 1,3,5-triphenylbenzene (TPB) skeleton with different peripheral modifications were purposefully designed and synthesized by tuning the ratio of p-type carbazole units and n-type diphenylphosphine oxide (DPPO) units from 0 : 3 to 3 : 0. Their optical, electrochemical and thermal properties were investigated systematically. By gradually tuning the D : A ratio of the peripheral groups, the desired ambipolar characteristics were achieved by DCzPOPB and CzDPOPB. As a result, the blue phosphorescent organic light-emitting diode (PhOLED) fabricated with the hosts doped with FIrpic achieved a low turn-on voltage of 3.3 V, a maximum current efficiency of 32.2 cd A−1, a maximum external quantum efficiency of 16.5%, a maximum luminescence of 22 440 cd m−2, as well as a low efficiency roll-off. Compared to the unipolar analogues, the device performances were significantly improved. In addition, the systematic study of the varied ratios of DPPO and carbazole units revealed the structure–property relationships of these starburst molecules, and a new avenue is proposed for the design of bipolar hosts for blue PhOLEDs.

 Please wait while we load your content...
Please wait while we load your content...