A novel benzothiazole-based enaminone as a fluorescent probe for highly selective and sensitive detection of CN−†
Abstract
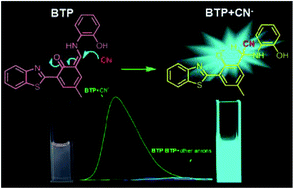
A novel benzothiazole derived enaminone BTP as a fluorescent probe for CN− recognition has been developed. In DMSO/H2O (95/5, v/v, HEPES 10 mM, pH = 7.4) solution, BTP exhibits high selectivity toward CN− among various anions through colorimetric and fluorescence dual channel changes. On treatment with CN−, BTP displays a blue shifted fluorescence enhancement and a naked eye observable color change from orange to yellow green. Moreover, the CN− sensing event possesses an excellent anti-interference ability over other anions. Sensing mechanism investigations prove that the CN− recognition process undergoes a typical nucleophilic addition of CN− to the enaminone formed BTP. Simple test paper experiments evidenced the practicability of BTP for CN− detection.

 Please wait while we load your content...
Please wait while we load your content...