Morphology evolution and the tri-continuous morphology formation of a PVDF/PS/HDPE ternary blend in melt mixing†
Yan Shao,
Rui Dou,
Shuang-lin Li,
Bo Yin* and
Ming-bo Yang
College of Polymer Science and Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu, Sichuan, People's Republic of China. E-mail: yinbo@scu.edu.cn; Fax: +86-28-85405324; Tel: +86-28-85405324
First published on 14th April 2016
Abstract
Tri-continuous morphology evolution and the formation of a PVDF/PS/HDPE system were studied in this paper. The blends were prepared by melt mixing using an internal mixer. The morphology was analyzed by scanning electron microscopy and optical microscopy. The evolution of the stable morphology would undergo three stages: firstly, most of PS dispersed in HDPE and then all of PS migrated into the interface of PVDF and HDPE; in the end, a portion of PS droplets moved into the HDPE phase and that is the stable morphology. Additionally, the effect of the PS volume fraction on the stable morphology was studied, and the results demonstrated that the PS volume fraction played only a little role on the sub-inclusion structure when the tri-continuous morphology was formed and the thickness of the PS layer shows a clear linear relationship with the content of PS; the viscosity ratio of HDPE/PS is of significant importance on the stable morphology as well as the continuity level of PS, when the viscosity ratio is greater than 1, the tri-continuous morphology cannot form, if the viscosity ratio is about 1, a perfect tri-continuous structure can be observed; when the viscosity ratio is less than 1, the morphology observed is combination of a tri-continuous morphology and sub-inclusion structure.
Introduction
Polymer blending is a very simple and cost-effective method for producing new materials from pure polymers.1–5 As is well known, the morphology of a polymer blend has a great influence on the physical–mechanical properties, so manipulation of phase morphology is very important.6–12 For binary blends, the morphological state is principally divided into a matrix-dispersed phase or a co-continuous morphology. On the other hand, ternary polymer blends can demonstrate a wide variety of micro-structured morphologies with multiple interfaces present and the morphology of ternary blends can be predicted.Generally, Harkins spreading equation is widely used to predicted type of wetting phenomenon in ternary polymer blends.13–15 Torza and Mason16 were the first who applied the Harkins equation into predicting two possible morphological categories, which include partial wetting and complete wetting in ternary mixtures by the calculation of spreading coefficients and then Hobbs etc.17 rewrote the Harkin's equation for a system in which two dissimilar polymers are dispersed within a third by substituting the appropriate interfacial tensions for the surface tensions values.
The spreading coefficients, is expressed by the following equation using the interfacial tension, γ:
 | (1) |
Recently most work are concentrated on core–shell phase morphology, both thermodynamic factors and kinetic factors were studied. Thermodynamic factors mainly involved in interfacial tension,18 free energy; kinetic factors primarily related to composition ratio,19 viscosity ratio20,21 and elastic effect.22 In a word, the core–shell phase morphology was systematically studied and this structure is fully applied into improving the properties.14,23–26
Tri-continuous morphology is the most complex structure of the complete wetting case where a continuous polymer (B) is situated at the interface of a co-continuous A/C system and all phases are highly continuous and percolated throughout the blend. As far as we know, only a few works are focused on tri-continuous morphology, Sepehr Ravati27 et al. determined the continuous region of PMMA/PS/HDPE system by triangular compositional diagrams; T. S. Omonov28 studied PA6/PP/PS system for a 40/30/30 composition, a three phase-co-continuous morphology was achieved. Adding compatibilizer precursors into this system, the morphology was modified deeply; Sepehr Ravati and Christine Beaulieu29 tailored the tri-continuous morphology in PBS/PLA/PBAT system to improve the mechanical properties, they found that since the property sets obtained in the final mixture can be more effectively shared from the three different polymers, the continuity of the components allows them to better contribute to the final properties.
Except for the excellent properties the tri-continuous morphology provides, owing to their interconnected nature, tri-continuous morphology has the potential to significantly widen the application range for polymer blend. They can be used in many fields: separation phenomena, electrical conductivity, tissue engineering scaffolds and drug delivery devices.30 Our previous work was focused on the functionalization of the tri-continuous morphology of PVDF/PS/HDPE system. Rui Dou31 et al. mixed carbon nanotube with PVDF/PS/HDPE system and find that carbon nanotube selectively dispersed in PS phase and has little influence on the morphology. In addition, they studied the properties of electrical conduction and electromagnetic interference shielding effectiveness. The results demonstrated that at a lower content of carbon nanotube, this system can reach the percolation threshold. In the process, we note that phase percolation is an important consideration. Therefore, controlling the index of continuity plays crucial role on the process of the function.
In the light of above reasons, it is indispensable to systematically study the tri-continuous morphology – the evolution of this structure and the manipulation of percolation threshold for the intermediate phase. This work can make preparation for the further work: the achievement of multiphase continuous morphology at the low concentration of intermediate phase; the functionalization of tri-continuous morphology; the establishment of the relationship between morphology and the properties.
This work mainly focus on the evolution of tri-continuous morphology in PVDF/PS/HDPE ternary blends under dynamic mixing conditions, in addition, kinetic factors: composition and viscosity ratio of HDPE/PS influencing continuity of interface phase are also studied.
Experimental
Materials
The key properties of these materials are shown in Table 1. Polyvinylidene fluoride (PVDF) FR906 was supplied by Shanghai 3F New Materials Company (China), two different viscosity polystyrene (PS): H-PS and L-PS were obtained from Taiwan QiMei company and Sanxing company, two different viscosity high-density polyethylene (HDPE): L-HDPE and H-HDPE were respectively from Fushun Petrochemical Company (China) and Lanzhou Petrochemical Company (China). Mw data of these polymers were obtained from the suppliers. The detail information of each component is notified in Table 1.| Matrix | Type | Mw (g mol−1) | ρ (g cm−3) at 200 °C | η (Pa s) at 50 s−1 |
|---|---|---|---|---|
| a H-PS, H-HDPE respectively represents higher viscosity PS and HDPE; L-PS, L-HDPE respectively represents lower viscosity PS and HDPE. | ||||
| PVDF | FR906 | 3.70 × 105 | 1.6 | 145.1 |
| H-PS | PG-33 | 1.74 × 105 | 0.95 | 322.6 |
| L-PS | 1810 | 1.53 × 105 | 0.95 | 206.2 |
| H-HDPE | 5000S | 3.18 × 105 | 0.85 | 905.1 |
| L-HDPE | 2911 | 1.70 × 105 | 0.85 | 227.5 |
Sample preparation
PVDF and PS were dried in a vacuum oven for 24 h at 80 °C before blending to minimize the effects of moisture. Binary and ternary blends were obtained by mixing the components of the blend in Haake torque mixer at a temperature of 200 °C with a rotor speed of 100 rpm for 8 min. Using the technique described by Bousmina et al.32 the average shear rate in the mixer was estimated to be about 50 s−1.The binary blends were obtained to infer the interfacial tension between their components using rheological measurements. The binary blends were obtained in a 80/20 composition with mixing time for 8 min.
To study the evolution of tri-continuous morphology, several samples of the ternary blend for PVDF/H-PS/L-HDPE (in a 45/20/35 composition) were prepared as a function of processing time. In the first step, PVDF and HDPE were mixed together for 3 min (necessary time to obtain co-continuous morphology), in the second step, PS was added into this mixture and mixing was continued further for 30 S, 1 min, 2 min, 3 min, 5 min, 8 min.
Interfacial tension measurement
Several methods can be used to evaluate the interfacial tension between molten polymers.33 In this work, the interfacial tension for the pairs of polymers (PVDF/PS, PVDF/HDPE and PS/HDPE) was determined using the rheological behaviour of their respective blend. The data were analysed using Gramespasher and Meissner's34 analyses following the procedures reported elsewhere.35 The results concerning the interfacial tensions are listed in Table 2 and the spreading coefficients calculated by eqn (1) are shown in Table 3.| Polymer pairs | Interfacial tension (mN m−1) |
|---|---|
| PVDF/PS | 9.7 |
| PS/HDPE | 0.8 |
| HDPE/PVDF | 13.5 |
| Spreading coefficient (mN m−1) | |
|---|---|
| λ(PS/HDPE) | 3 |
| λ(PVDF/PS) | −22.4 |
| λ(HDPE/PVDF) | −4.6 |
Rheological characterization
Rheological characterization was carried out using an AR2000ex stress controlled dynamic rheometer (TA Corporation). The experiments were performed in parallel-plate geometry with a gap of about 1.3 mm under a nitrogen atmosphere at a temperature of 200 °C. Disks of 25 mm diameter and 1.5 mm thickness were compression moulded at 200 °C, under a pressure of 10 MPa for 3 min. Dynamic frequency sweeps were performed for pure components and the binary blends. The frequency was varied from 100 to 0.01 Hz and the strain amplitude was kept small enough (2%) to ensure a linear viscoelastic response of the polymers.Solvent extraction
In order to determine the continuity index of components in the blend, a solvent extraction/gravimetric method was performed. It is a fast and straightforward technique to detect the existence of continuous microstructures with a high accuracy when the components are soluble in specific solvents. Xylene was utilized as selective solvents for polystyrene. The samples were parallel plate geometry with 25 mm diameter and 1.5 mm thickness. The solvent extraction was performed at room temperature in small glass tubes for one week for PVDF/PS/HDPE blends over the entire composition range. After drying the samples in a vacuum oven at 80 °C, the sample was weighed. In the second step, samples were again subjected to fresh solvents for 2 days followed by drying and weighing. This procedure was repeated until the sample weight from two consecutive extractions remained unchanged. In this approach, the volumes of the components before and after extraction are measured by weighing the sample and converting the weight to volume.
 | (2) |
Characterization of phase morphology
The morphologies of all the blends were characterized by a JEOL JSM-5900LV scanning electron microscopy (SEM, JEOL, Japan) at a 20 kV accelerating voltage. The samples were frozen in liquid nitrogen for 40 minutes. In order to create a perfect plane face, the specimens were microtomed using a Reichert Jung 2050 Supercut Microtome. The fractured surfaces were sputtered with gold before observation. To accentuate the contrast between phases, PS phase was etched with xylene and PVDF was etched with DMF.The morphologies were also observed by Olympus BX51 polarizing optical microscopy (Olympus Co., Tokyo, Japan) under both bright field and crossed-polar conditions. The specimens were prepared into 30 μm thick sections by a microtome.
Quantitative analysis of the morphology was performed using image analysis of Image-Pro Plus 6. At least 500 PS dispersed domains were measured by manually tracing the phase boundaries to estimate the thickness of PS phase when the influence of volume fraction of PS on the continuity was studied.†
Results and discussion
The prediction of morphology for PVDF/PS/HDPE ternary
For PVDF/PS/HDPE ternary blends, the very high interfacial tension of PVDF/HDPE, the low interfacial tension of PS/HDPE and PVDF/PS result in a positive spreading coefficient of PS over PVDF (PS/HDPE) with a value of 3 mN m−1. The other two spreading coefficients are negative: λ(PVDF/PS) = −22.4 mN m−1 and λ(HDPE/PVDF) = −4.6 mN m−1. This predicts a complete wetting case with the development of a thermodynamically stable PS layer between PVDF and HDPE.Fig. 1 presents the phase morphology of PVDF/PS/HDPE ternary blends with a composition ratio of 45/20/35 vol%. The cryo-fracture surface (Fig. 1a) shows that all phases are percolated and a tri-continuous microstructure is produced. To identify which phase is located where, PS phase and PVDF phase were etched respectively. It is noted that uniform cracks spread out the sample entirely (Fig. 1b), when PS phase is etched by xylene; Fig. 1c shows continuous HDPE phase after extraction of PVDF and PS. However, from the SEM micrographs, a significant fraction of the PS droplets residing inside the HDPE phase can be seen, because of the low interfacial tension of HDPE/PS polymer pair. Similar results was observed in the tri-continuous morphology of PMMA/PS/HDPE system30 and the partial wetting morphology of PP/PS/HDPE system.36 Additionally, crystalline structure of samples under polarized optical microscopy also shows that a total continuous structure of different phase is formed (Fig. 1d and e). Owing to different melting point of PVDF and HDPE, it is possible to distinguish the phases, which are marked in Fig. 1d and e.
Prior to polarized optical microscopy analysis of the ternary blend, wide-angle X-ray diffraction (WAXS) patterns† (Fig. S1) and DSC heating curves† (Fig. S2†) of pure PVDF, HDPE and PVDF/PS/HDPE ternary blend were obtained, because WAXS and DSC data can provide a convenient way of characterizing the contribution of the individual components to the blends.28,37,38 The results of WAXS and DSC demonstrated that blending has no significant effect on the type of crystals in our PVDF/PS/HDPE system, so optical microscopy can be used to distinguish the phases successfully.
Blend morphology of PVDF/PS/HDPE ternary blend
 | ||
| Fig. 3 Schematic diagram of the evolution of tri-continuous morphology for PVDF/PS/HDPE system; (yellow is the PVDF phase; blue is the HDPE phase; black is PS). | ||
Because of its 2D nature, SEM does not always provide the complete information about the blend phase morphology. Therefore, quantitative extraction experiments were performed as well. Fig. 4 shows the level of continuity of PS phase. From the result, we can know that when mixing 2 min, the continuity level of PS phase is highest, which verifies the observed phenomenon that it forms perfect tri-continuous morphology at this mixing time. When the mixing time increases, the continuity reduces. It is also anastomotic with the SEM and optical micrographs.
Analysing the evolution of tri-continuous morphology, during the initial stage of mixing, the morphology is mostly influenced by the thermodynamic factor: interfacial tension. The interfacial tension of HDPE/PS is 0.8 mN m−1, which is less than the one of PVDF/HDPE; it indicates that the affinity between PS and HDPE is better than PS and PVDF, so when the mixing time is shorter, most of PS dispersed in HDPE. However, with the mixing time increasing, kinetic factors may play a dominant role. At the driving force of shear stress, all of PS mobile into interface, but part of PS phase relocated on HDPE phase when the mixing time is prolonged. It can be attributed to the following reasons: the first one, owing to the immiscible property of PVDF/PS/HDPE system, the interface is not stable, at the driving force of shear stress, a number of PS droplets transfer into HDPE phase; the second one, in the process of mixing, the droplets would rupture and coalescence, which result in increasing or decreasing of the size of phase. From the SEM, we can see that the phase domain would increase which lead to the decrease of the interfacial area, in result, a percentage of PS would migrate into HDPE phase. Moreover, concentration of PS and the viscosity ratio of HDPE/PS may also influence the steady morphology: most of PS situates in the interface with some isolated PS droplets locating in HDPE.
To further enhance the factors on the steady structure and the continuity level of PS phase, concentration of PS and the viscosity ratio of HDPE/PS were performed, as we will show in next sections.
Lots of published work has reported that saturated interface is existent. Sepehr Ravati found that as the concentration of PS is increased, more isolated droplets are located in the PMMA since the HDPE/PMMA interface is already saturated by PS,27 when they studied tri-continuous morphology in HDPE/PS/PMMA system. In another line of work, the thickness of shell has also been investigated in core–shell structure, shell thickness do not change along with content of shell phase.39,40
In order to verify our supposition, the following experiments were made: under the condition of PVDF/HDPE is constant (volume ratio), volume fraction of PS varies from 10% to 40%. The samples were obtained in Haake torque mixer at a temperature of 200 °C with a rotor speed of 100 rpm for 8 min.
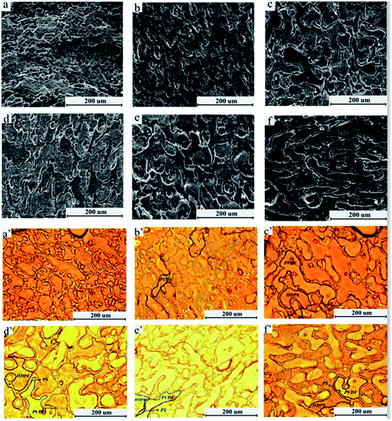
Fig. 5 demonstrates the dependence of the morphology on the volume fraction of PS (vol%) at 10, 12, 16, 20, 24, 28, 32, 36, and 40. The SEM morphologies can be classified into three categories: type I, type II and type III. The first one: combination of core–shell structure (PVDF is core, PS is shell) and tri-continuous morphology, as is shown in Fig. 5a; the second: most of PS phase situates in the interface with some isolated droplets locating in HDPE, which can be seen from Fig. 5b–g, when the volume fraction of PS is 12–32%; in type III, as the concentration of PS is increased (volume fraction of PS exceeds 32%), tri-continuous morphology is not formed, phase inversion occurred: HDPE phase transfer into PS phase. The schematic diagram was shown in Fig. 6.
 | ||
| Fig. 5 SEM micrographs as a function of PS fraction volume; (a) 10% PS; (b) 12% PS; (c) 16% PS; (d) 20% PS; (e) 24% PS; (f) 28% PS; (g) 32%; (h) 36%; (i) 40% (all of PS phase was etched by xylene). | ||
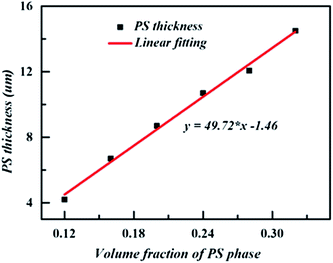
To better understand the effect of the volume fraction of PS on the sub-inclusion morphology, the thickness of PS of tri-continuous morphology were estimated, the results are shown in Fig. 7. Clearly, and not unexpectedly, the thickness of PS phase increases with the content of PS, saturated closely-packed layer is not existent in our system. The results are not in accordance with previous work.27,39,40 From the result, we can know that the interface tension has subtle influence on the sub-inclusion morphology and it does not play a dominant role as we predicted.
Two types HDPE and PS were chosen and the properties were shown in Table 1, the viscosity ratio (HDPE/PS) of these systems are respectively more than 1; about 1 or less than 1 at the shear rate of 50 s−1. The complex viscosity as a function of shear rate for the raw materials used is shown in Fig. 8 and the viscosity ratio of HDPE/PS corresponding to the shear stress are shown in Table 4. All these three types ternary blends were obtained at a 45/20/35 (volume fraction) composition in a Haake torque mixer at a temperature of 200 °C with a rotor speed of 100 rpm for 8 min.
 | ||
| Fig. 8 Complex viscosity versus function of frequency for the pure polymers at 200 °C. (The dashed line represents the average shear rate in the mixer). | ||
| Composition | Viscosity ratio of HDPE/PS |
|---|---|
| PVDF/H-PS/H-HDPE | 2.8 |
| PVDF/L-PS/L-HDPE | 1.1 |
| PVDF/H-PS/L-HDPE | 0.5 |
The influence of viscosity ratio of HDPE/PS on the sub-inclusion is shown in Fig. 9. It is clearly illustrated that a change in the viscosity ratio of HDPE/PS for the same blend composition has significant effect on the blend phase morphology. For a high viscosity ratio of HDPE/PS, this system cannot form tri-continuous morphology; PS does not coalesce into a uniform layer at the interface of PVDF and HDPE, which can be observed in Fig. 9a. Optical micrograph also displays that tri-continuous morphology is not formed in this system (Fig. 9a′ and a′′). The reasons for this can be explained are as follows. From the evolution of tri-continuous morphology, one can know that PS phase would first dispersed in HDPE and then migrate into interface of PVDF and HDPE at the driving force. When the viscosity ratio of HDPE/PS is higher, owing to the highly viscous HDPE, PS droplets migrated to interface would sustain more resistance, so it is difficult for PS to migrate to the interface of PVDF and HDPE. Certainly, the immobilization of PS sub-inclusions in HDPE is also encouraged by the relatively low PS/HDPE interfacial tension. Similar results have been reported by Joel Reignier.22 They studied the core–shell structure of HDPE/PMMA/PS system and found the presence of a high level of shell sub-inclusion material in a highly viscous core of the composite droplet. They also attributed this into the restriction of high viscous core.
In the case of viscosity ratio of HDPE/PS is about 1 or less than 1, both of the systems can form tri-continuous morphology, distinct interface can be seen in optical micrographs (Fig. 9b′, b′′, c′ and c′′). Fig. 9b manifests a perfect tri-continuous morphology, in which all of PS dispersing in the interface when the viscosity of HDPE and PS is similar. In addition, the continuity of PS for this system is 100% ± 2, which also verifies above results.
It should be noted that some isolated PS droplets are located in HDPE phase as is shown in Fig. 9c, when the viscosity ratio of HDPE/PS is less than 1. This can be confirmed by the solvent extraction result of PS phase, continuity of PS phase is 93.3%, which means about 7% of PS encapsulated by HDPE. Similar results also have been reported by V. Everaert et al.20 When studying POM(PS/PPE) system, they also find complex morphology. As demonstrated by them, the difference in softening temperature between both components can lead to this phenomenon. In the system of low viscosity ratio of HDPE/PS, the viscosity of HDPE is lower than PS and the glass transition temperature of HDPE is −120 °C, which is also lower than 116 °C of PS. In the mixing process, the lower melting component – HDPE will always first encapsulate the higher softening component – PS; in addition, according to general concept of some semi-empirical models that the less viscous phase tends to encapsulate the more viscous one,41 so the low viscosity HDPE would encapsulate the high viscosity PS. Based on above reasons, with the mixing time increasing, although most PS would merger and migrate to the interface of PVDF and HDPE, there is a little PS still being encapsulated by HDPE.
Clearly, the control of viscosity ratio for HDPE/PS is a very promising approach to achieve perfect tri-continuous morphology!
Conclusions
This study researched the evolution of tri-continuous morphology of PVDF/PS/HDPE ternary blend. The evolution of the stable morphology as a function of mixing time can be divided into three stages: firstly, a large quantity of PS was encapsulated by HDPE, which can be attributed to the low interfacial tension of PS and HDPE; then, all of PS migrated to the interface of PVDF and HDPE, which may be caused by the large shear stress; in the end, a part of PS migrate to HDPE phase and that is the stable morphology.In the case of PS volume fraction, the results demonstrate that the volume fraction of PS has little influence on the sub-inclusion. What's more, we find that the thickness of PS layer increases with the content of PS; a spectacular transition occurs when changing viscosity ratio of HDPE/PS. Perfect tri-continuous structure is formed when the viscosity ratio is about 1; if viscosity ratio is less than 1, the morphology is observed as tri-continuous morphology and sub-inclusion structure; however, when the viscosity of HDPE is much greater than PS, they cannot form tri-continuous morphology.
Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Contract No. 51273219, 51573106 and 51421061), the National Key Basic Research Program of China (973 Program, No. 2012CB025902), the Foundation of State Key Laboratory of Polymer Materials Engineering (Grant No. sklpme2014-3-12) and the Fundamental Research Funds for the Central Universities (No. 2013SCU04A03).References
- C. Mao, Y. Zhu and W. Jiang, ACS Appl. Mater. Interfaces, 2012, 4, 5281–5286 CAS.
- B. Yin, L.-p. Li, Y. Zhou, L. Gong, M.-b. Yang and B.-h. Xie, Polymer, 2013, 54, 1938–1947 CrossRef CAS.
- G. P. Kar, S. Biswas, R. Rohini and S. Bose, J. Mater. Chem. A, 2015, 3, 7974–7985 CAS.
- J. Gao, J. Li, S. Zhao, B. C. Benicewicz, H. Hillborg and L. S. Schadler, Polymer, 2013, 54, 3961–3973 CrossRef CAS.
- H. Rastin, S. H. Jafari, M. R. Saeb, H. A. Khonakdar, U. Wagenknecht and G. Heinrich, J. Polym. Res., 2014, 21, 1–13 CAS.
- A. Dasari, Q.-X. Zhang, Z.-Z. Yu and Y.-W. Mai, Macromolecules, 2010, 43, 5734–5739 CrossRef CAS.
- H. Liang, B. Favis, Y. Yu and A. Eisenberg, Macromolecules, 1999, 32, 1637–1642 CrossRef CAS.
- G. Zhong, K. Wang, L. Zhang, Z.-M. Li, H. Fong and L. Zhu, Polymer, 2011, 52, 5397–5402 CrossRef CAS.
- N. Nemirovski, A. Siegmann and M. Narkis, J. Macromol. Sci., Part B: Phys., 1995, 34, 459–475 CrossRef.
- T. Zhao, C. Zhang, Z. Du, H. Li and W. Zou, RSC Adv., 2015, 5, 91516–91523 RSC.
- R. Dou, S. Li, Y. Shao, B. Yin and M.-B. Yang, RSC Adv., 2016, 6, 439–447 RSC.
- L.-p. Li, B. Yin, Y. Zhou, L. Gong, M.-b. Yang, B.-h. Xie and C. Chen, Polymer, 2012, 53, 3043–3051 CrossRef CAS.
- N. Virgilio, P. Desjardins, G. L'Espérance and B. D. Favis, Polymer, 2010, 51, 1472–1484 CrossRef CAS.
- A. Wilkinson, M. Clemens and V. Harding, Polymer, 2004, 45, 5239–5249 CrossRef CAS.
- P. Le Corroller and B. D. Favis, Polymer, 2011, 52, 3827–3834 CrossRef CAS.
- S. Torza and S. Mason, J. Colloid Interface Sci., 1970, 33, 67–83 CrossRef CAS.
- S. Hobbs, M. Dekkers and V. Watkins, Polymer, 1988, 29, 1598–1602 CrossRef CAS.
- A. Legros, P. Carreau, B. Favis and A. Michel, Polymer, 1997, 38, 5085–5089 CrossRef CAS.
- I. Luzinov, K. Xi, C. Pagnoulle, G. Huynh-Ba and R. Jérôme, Polymer, 1999, 40, 2511–2520 CrossRef CAS.
- V. Everaert, G. Groeninckx and L. Aerts, Polymer, 2000, 41, 1409–1428 CrossRef CAS.
- J. Reignier, B. D. Favis and M.-C. Heuzey, Polymer, 2003, 44, 49–59 CrossRef CAS.
- J. Reignier and B. D. Favis, AIChE J., 2003, 49, 1014–1023 CrossRef CAS.
- J. Chen, A. Kinloch, S. Sprenger and A. Taylor, Polymer, 2013, 54, 4276–4289 CrossRef CAS.
- K. Wang, Y. Chen and Y. Zhang, Polymer, 2009, 50, 1483–1490 CrossRef CAS.
- C. Shen, Y. Zhou, R. Dou, W. Wang, B. Yin and M.-b. Yang, Polymer, 2015, 56, 395–405 CrossRef CAS.
- R. Dou, C. Shen, B. Yin, M.-b. Yang and B.-h. Xie, RSC Adv., 2015, 5, 14592–14602 RSC.
- S. Ravati and B. D. Favis, Polymer, 2010, 51, 4547–4561 CrossRef CAS.
- T. S. Omonov, C. Harrats and G. Groeninckx, Polymer, 2005, 46, 12322–12336 CrossRef CAS.
- S. Ravati, C. Beaulieu, A. M. Zolali and B. D. Favis, AIChE J., 2014, 60, 3005–3012 CrossRef CAS.
- J. Zhang, S. Ravati, N. Virgilio and B. D. Favis, Macromolecules, 2007, 40, 8817–8820 CrossRef CAS.
- R. Dou, Y. Shao, S. Li, B. Yin and M. Yang, Polymer, 2016, 83, 34–39 CrossRef CAS.
- M. Bousmina, A. Ait-Kadi and J. Faisant, J. Rheol., 1999, 43, 415–433 CrossRef CAS.
- N. R. Demarquette, A. M. C. De Souza, G. Palmer and P. H. P. Macaubas, Polym. Eng. Sci., 2003, 43, 670–683 CAS.
- H. Gramespacher and J. Meissner, J. Rheol., 1992, 36, 1127–1141 CrossRef CAS.
- P. Macaubas and N. Demarquette, Polymer, 2001, 42, 2543–2554 CrossRef CAS.
- N. Virgilio, C. Marc-Aurele and B. D. Favis, Macromolecules, 2009, 42, 3405–3416 CrossRef CAS.
- S. Ross, P. D. Topham and B. J. Tighe, Polym. Int., 2014, 63, 44–51 CrossRef CAS.
- R. Dou, W. Wang, Y. Zhou, L. p. Li, L. Gong, B. Yin and M. b. Yang, J. Appl. Polym. Sci., 2013, 129, 253–262 CrossRef CAS.
- T. S. Valera, A. T. Morita and N. R. Demarquette, Macromolecules, 2006, 39, 2663–2675 CrossRef CAS.
- J. Reignier and B. D. Favis, Macromolecules, 2000, 33, 6998–7008 CrossRef CAS.
- R. Tol, G. Groeninckx, I. Vinckier, P. Moldenaers and J. Mewis, Polymer, 2004, 45, 2587–2601 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra07877j |
| This journal is © The Royal Society of Chemistry 2016 |