Modified photoanodes by amino-containing phosphonate self-assembled monolayers to improve the efficiency of dye-sensitized solar cells
Chun-Chu Lin and
Chun-Pei Cho*
Department of Applied Materials and Optoelectronic Engineering, National Chi Nan University, Nantou County 54561, Taiwan. E-mail: cpcho@ncnu.edu.tw
First published on 16th May 2016
Abstract
Surface modification of TiO2 electrodes by selected molecules could lower the energy barrier of electron transfer and improve DSSC performance. The category of the terminal group and molecular length of a self-assembled monolayer influence the surface and interfacial properties of a TiO2 electrode, and the photovoltaic parameters of a DSSC could thereby be adjusted. By electrochemical approaches, it was discovered that the redox current and photocurrent increased when a lower work function and thus a reduced impedance at the TiO2/dye/electrolyte interface were achieved by using an amino-containing phosphonic acid. However, a smaller photocurrent would be caused when the path for electron transport and charge recombination probability were increased by employing a longer molecule. Both the photocurrent and dark current of a DSSC were suppressed when a larger impedance restrained electron transport through the interface. The TiO2 electrode modified by 2-aminoethylphosphonic acid showed the largest redox current. The corresponding DSSC exhibited the smallest impedance, largest photocurrent and highest efficiency of 6.67%. This study has demonstrated that a monolayer formed on the TiO2 surface by an amino-containing phosphonic acid enhanced DSSC performance efficiently.
1. Introduction
Dye-sensitized solar cells (DSSCs), belonging to photoelectrochemical systems, are currently the most efficient third-generation solar technology available. Based on nanocrystalline high bandgap semiconductors such as titanium dioxide (TiO2), they have been regarded as one of the most promising and economical alternatives to the silicon-based counterparts since O'Regan et al. reported the breakthrough of a high energy conversion efficiency (η).1 Afterwards lots of people have started to devote efforts to this research area. Up to now the highest η of DSSCs (not including those using perovskite materials) ever reported has reached 12%.2 There are several major research directions to improve DSSC performance: changes of anode structure, electrode material, molecular structure of dye, category of electrolyte, and application of various substrates, etc.3–7 Although the η is less than the best thin-film cells at present, such kind of device is still worthy for more explorations because it owns the advantages such as simple processes, low cost, large spectral range of light absorption, light absorption on dual sides, compatibility of flexible substrates, and the potential for large size and mass production.TiO2 is the most common semiconductor material employed to fabricate anode of DSSCs because of its good stability, inexpensiveness, easy availability and no light corrosion. According to the literature, the larger bandgap of anatase TiO2 can prevent photogenerated electrons in the conduction band from going back to the valence band. Furthermore, it was reported that anatase TiO2 has a larger specific surface area for dye adsorption.8 Increasing studies have been concerning approaches for surface modification on TiO2 electrode. Wu et al. coated an aluminium oxide (Al2O3) thin layer with a controlled thickness on TiO2. Dye adsorption was increased and charge recombination was inhibited, and η was thereby enhanced.9 Wang et al. utilized titanium tetrachloride (TiCl4) solution to grow rutile nanorods on TiO2 surface to increase the specific surface area and amount of dye adsorption. The optimum TiCl4 concentration and growth time could lead to a higher η.10 It was consequently perceived that an appropriate approach to modifying TiO2 electrode could contribute to improved DSSC performance.
The formation of self-assembled monolayers (SAMs) is ascribed to spontaneous chemical adsorption of gaseous or liquid organic molecules. By self-assembly manner, a monolayer with stable orderly arrangement and compact structure completely covers the substrate surface. However, no much attention was paid to SAMs until disulfide monolayers on gold (Au) were reported by Nuzzo et al.11 The processes required for preparing SAMs are simple. They have provided a facile way to adjust the surface characteristics of a substrate. Accordingly, they can be applied to various areas such as surface modifications of substrates (electrodes, nanoparticles or films), chemi- or bio-sensors, electrochemical applications, DNA immobilization, and manufacture of nanomaterials, etc.12–18
Carboxylic and phosphonic acids can form orderly arranged SAMs on metal or metal oxide surfaces.19–21 Tao et al. utilized n-alkanoic acids with various chain lengths to form carboxylate SAMs on the surfaces of noble metals and analyzed their structures by ellipsometry and reflection absorption infrared spectroscopy.22 Breen et al. used alkylphosphonic acids to fabricate patterns on transparent metal oxide thin films by microcontact printing and wet etching.23 Robel et al. used thiol-containing organic acids to modify TiO2 surface to obtain modified cadmium selenide (CdSe) quantum dots.24 There has been literature regarding surface modification of TiO2 by SAMs. Moreover, it has been illustrated that stronger bonds would form between phosphonic acids and TiO2 compared to carboxylic acids.25,26 With regards to this, higher DSSC stability is supposed to be achieved if phosphonic acids rather than carboxylic acids are used to form SAMs on TiO2 electrodes. In our previous study, several phosphonic acids with various terminal groups and molecular lengths were chosen to alter the work function of TiO2 to see how dipole moment affected DSSC performance. A higher η was achieved as TiO2 was modified by an amino-containing phosphonic acid.27 Herein, similar organic acids were also selected. The surface potential and interfacial characteristics of a photoanode could be thereby regulated. Electrochemical analysis and discussion were emphasized in this work. The impacts of using different SAMs on the photovoltaic parameters and DSSC performance were explored and compared by various electrochemical methods.
2. Experimental
2.1 Fabrication of photoanodes
Commercial conductive glasses covered with fluorine-doped tin oxide (FTO, 7 Ω cm−2) were used as the substrates for fabrication of photoanodes. The FTO glasses were firstly cleaned with detergent, rinsed twice with de-ionized water, followed by ultrasonication in ethanol and isopropyl alcohol baths sequentially, and finally dried by high nitrogen gas flow to obtain clean FTO substrates. The nanoporous electrode was composed of three-layer TiO2 nanoparticles. The diameters of the nanoparticles are 13 nm in the first two layers (Ti-Nanoxide T/SP, Solaronix) and 400 nm in the uppermost layer (Ti-Nanoxide R/SP, Solaronix). The nanoparticles of each layer were spread on clean FTO glasses by doctor blade method using adhesive tape as the spacer, followed by air-drying at room temperature and baking at 100 °C in an oven for 2 hours, respectively, and then calcination at 500 °C for 60 min.The TiO2 electrode with an effective area of 0.5 × 0.5 cm2 was immersed in the 0.3 mM dye solution (N719 in t-butanol/acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v)) for 24 hours. Then the dye-anchored TiO2 electrode was drawn out from the dye solution and rinsed with acetonitrile repeatedly. Afterward, it was blow-dried with high nitrogen gas, followed by immediate immersion in the 1 mM solutions of selected chemicals for 18 hours. Seven commercially available chemicals with different terminal groups and molecular lengths were employed for surface modification to form monolayers (SAMs). They are AEPA (2-aminoethylphosphonic acid), ABPA (4-aminobutylphosphonic acid), BPA (1-butylphosphonic acid), HPA (n-hexylphosphonic acid), PA (phosphoric acid), FA (formic acid) and NH3 (ammonia, 2.0 M solution in ethanol), as listed in Table 1. Their chemical structures are displayed in Fig. 1. The solvent used to dissolve AEPA and ABPA was a mixture of acetone and toluene (1
1, v/v)) for 24 hours. Then the dye-anchored TiO2 electrode was drawn out from the dye solution and rinsed with acetonitrile repeatedly. Afterward, it was blow-dried with high nitrogen gas, followed by immediate immersion in the 1 mM solutions of selected chemicals for 18 hours. Seven commercially available chemicals with different terminal groups and molecular lengths were employed for surface modification to form monolayers (SAMs). They are AEPA (2-aminoethylphosphonic acid), ABPA (4-aminobutylphosphonic acid), BPA (1-butylphosphonic acid), HPA (n-hexylphosphonic acid), PA (phosphoric acid), FA (formic acid) and NH3 (ammonia, 2.0 M solution in ethanol), as listed in Table 1. Their chemical structures are displayed in Fig. 1. The solvent used to dissolve AEPA and ABPA was a mixture of acetone and toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Other five chemicals were dissolved in toluene. The modified TiO2 was then rinsed by acetonitrile, blow-dried with high nitrogen gas, and used as a photoanode in the fabrication of DSSCs immediately after being taken out from the solutions.
1, v/v). Other five chemicals were dissolved in toluene. The modified TiO2 was then rinsed by acetonitrile, blow-dried with high nitrogen gas, and used as a photoanode in the fabrication of DSSCs immediately after being taken out from the solutions.
| Dipole moment (debye) | Work function (eV) | |
|---|---|---|
| STD | 5.22 | |
| AEPA | −1.70 | 5.17 |
| ABPA | −1.94 | 5.16 |
| BPA | −1.47 | 5.30 |
| HPA | −1.49 | 5.28 |
| PA | −1.52 | 5.26 |
| FA | −1.51 | 5.27 |
| NH3 | −1.55 | 5.26 |
2.2 Fabrication of counter electrode (CE)
The FTO conductive glasses for fabricating platinum (Pt) CE were also cleaned by same processes as described above. A Pt ultra-thin film was deposited on the clean substrates by an ion sputter coater in vacuum with the sputtering time of 40 s and a deposition rate of 0.75 Å s−1.28 When the sputtering deposition was completed, the Pt CE was taken to calcination at 500 °C for 60 min. After cooling down to the room temperature, colloidal silver paste (Ted Pella, Inc.) was smeared over the edge of Pt CE to increase conductivity. Once the paste was air-dried, the CE could be employed in the fabrication of DSSCs.2.3 Fabrication of DSSCs
The DSSCs were of a sandwich type composed of a dye-adsorbed TiO2 electrode, a polyimide spacer and a CE which was a platinized FTO conductive glass. The electrolyte solution prepared from 0.5 M LiI (lithium iodide), 0.05 M I2 (iodine), 0.5 M TBP (tert-butylpyridine) and 0.5 M MPII (1-methyl-3-propylimidazolium iodide) in acetonitrile was injected into the narrow space between the two electrodes by a microsyringe. Alignment of the relative positions of two electrodes was needed so that a simple encapsulation could be performed to avoid leakage of the electrolyte. The standard DSSC with the unmodified TiO2 electrode was named as STD.2.4 Characterizations
The absorption spectra ranging from 250 nm to 600 nm of the dye-desorbed solutions obtained by immersing the SAM-modified TiO2 electrodes in 0.1 M NaOH (sodium hydroxide) aqueous solution were measured by a UV-vis spectrophotometer (U-3900H, Hitachi). To compare the amounts of dye adsorption, the absorbances in the UV- and visible-light regions were examined. The photovoltaic parameters of all DSSCs were obtained by a solar simulator (SS50ABA, Photoemission Tech, Inc.) under AM 1.5 filter irradiation. The photocurrent–voltage (I–V) characteristics were recorded by a source monitor coupled with the I–V measurement software under the constant light intensity of 100 mW cm−2. The approaches for electrochemical analysis including cyclic voltammetry (CV), chronoamperometry (CA) and electrochemical impedance spectroscopy (EIS) were carried out by a potentiostat/galvanostat analyzer (CHI627C, CH Instruments).3. Results and discussion
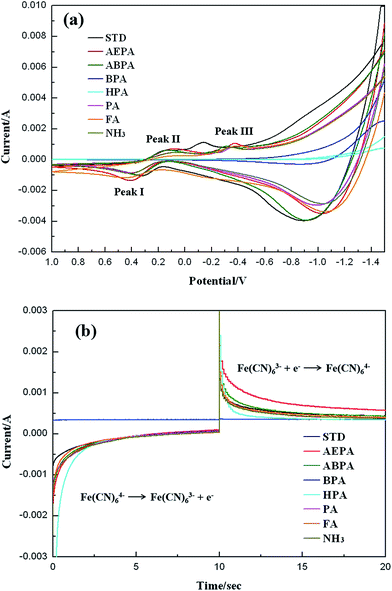
It was not easy to calculate the dipole moments of the monolayers adsorbed on TiO2 surfaces. Accordingly, those of single free molecules were calculated, compared and correlated to the work functions of various SAM-modified TiO2 electrodes. It was believed that the differences in dipole moments were mainly ascribed to the terminal groups. Thus the use of dipole moments of single free molecules could approximately reflect the relative dipole variations among the seven monolayers. In this study, the dipole moments are computed by PC Spartan Pro using the semi-empirical PM3 method with geometry optimization, and the work functions of the SAM-modified TiO2 electrodes are obtained by Kelvin probe, as listed in Table 1. It reveals that work function varies when the magnitude of dipole moment is different. Due to the stronger electron donating ability of an amino group, AEPA and ABPA have larger negative dipole moments, resulting in lower work functions of the AEPA- and ABPA-modified TiO2 electrodes. A lower work function would decrease the potential barrier and increase the rate of electron transport from dye to photoanode.27 The charge recombination probability was thereby reduced, and a larger photocurrent and higher DSSC performance would be achieved.To examine the impacts of surface modification on photoelectrochemical activity, CV tests were performed on the SAM-modified TiO2 electrodes. The electrolyte used for CV was an aqueous solution consisting of 0.1 M KCl and 5 mM K4Fe(CN)6. As can be seen in Fig. 2a, the CV curves show one oxidation and two reduction peaks labeled as peak I, II and III, respectively. According to the literature,29 the three peaks correspond to occurrence of the following reactions:
| Peak I: Fe(CN)64− → Fe(CN)63− + e− | (1) |
| Peak II: Fe(CN)63− + e− → Fe(CN)64− | (2) |
| Peak III: Ti4+ + e− → Ti3+ | (3) |
After magnification of the CV curves, it is discovered that the AEPA-modified TiO2 electrode exhibits the largest redox current, followed by the ABPA-modified and STD electrodes. By contrast, the BPA- and HPA-modified electrodes exhibit the smallest redox current. Generally, a larger redox current stands for more efficient electron transport and reduced charge recombination at the interface, and the photocurrent density (JSC) and η would thereby be enhanced. It is then speculated that the DSSC with AEPA-modified TiO2 electrode would show the highest JSC and η. Since there is no oxidation or reduction peak in the CV curves of BPA- and HPA-modified electrodes, it can be deduced that the DSSCs with the two electrodes would show the worst photovoltaic characteristics.
CA is one of the most commonly used electrochemical methods to examine the correlation between current response and time. When the applied potential is large enough to cause an electrochemical reaction, current will fluctuate over time. The CA curves of various SAM-modified TiO2 electrodes have been shown in Fig. 2b. The electrolyte used for CA is the same as that for CV. The oxidation and reduction reactions are the same as eqn (1) and (2), respectively. When the redox currents are listed by sequence of magnitude, i.e., AEPA > STD > ABPA > PA or FA or NH3 > HPA > BPA, it can be found that the trend is consistent with that of CV results. A larger redox current in a CA curve also represents faster charge transport which would give rise to enhanced photocurrent and η of a DSSC. As revealed in Fig. 2b, the AEPA-modified electrode exhibits the largest redox current. Accordingly, the DSSC with AEPA-modified electrode is supposed to have the best device performance. The CV and CA results can be correlated with the magnitudes of work function. A larger redox current is obtained when there is a lower work function. However, an exception is found in the ABPA-modified electrode having the lowest work function. It is supposed to exhibit the largest redox current but actually shows a current even smaller than those of STD and the AEPA-modified electrodes. This probably can be ascribed to the longer molecular length of ABPA which causes a bit longer path for electron transport.
The interfacial properties of a material affecting its conductivity can be detected and simulated by EIS. Recently, the EIS method has been widely utilized to analyze the nature of charge transfer at the interfaces in electrochemical systems. Now it has become an indispensable tool for the complex structures in solar cells. Simulation can be performed using a simple equivalent circuit to better understand the phenomenon occurring at the interface in an electrochemical system. Fig. 3a shows the Nyquist plots obtained by EIS analysis on the DSSCs with various SAM-modified electrodes. The second semicircle in a Nyquist plot related to the charge transfer impedance at the TiO2/dye/electrolyte interface is the emphasis since investigation has been focused on photoanodes. According to the radius of a semicircle, the charge transfer rate on each interface of the DSSCs can be compared. It can be noticed that the second semicircles of the two DSSCs with BPA- and HPA-modified electrodes have much larger radii, indicating larger impedances at the TiO2/dye/electrolyte interfaces. The two devices also exhibit the smallest dark currents. Both photocurrent and dark current of a DSSC are suppressed when there is a larger impedance restraining electron transport at the interface.
 | ||
| Fig. 3 (a) Nyquist plots of the DSSCs with various SAM-modified TiO2 electrodes. (b) Magnification of the high-frequency region. | ||
Fig. 3b shows the magnification of the high-frequency region in Fig. 3a. By the results of Fig. 3a and b, the interfacial impedance can be listed from the smallest to the largest in sequence: AEPA < STD < ABPA < PA < NH3 < FA < HPA < BPA. The corresponding impedance values obtained by simulation on EIS spectra are 78 Ω, 80 Ω, 83 Ω, 85 Ω, 93 Ω, 98 Ω, 819 Ω and 2529 Ω. The second semicircle of the DSSC with AEPA-modified electrode has the smallest radius, implying a smaller impedance at the TiO2/dye/electrolyte interface of the device. A smaller interfacial impedance would contribute to more efficient electron transport and thereby enhanced photocurrent and device performance. This is why the DSSC with AEPA-modified electrode shows the highest JSC and η. Except for that with ABPA-modified electrode, the EIS results are roughly correlated with the magnitude of work function. A smaller interfacial impedance would be obtained when there is a lower work function. The trend is similar to the CV and CA results. The impedance of the DSSC with ABPA-modified electrode is supposed to be the smallest. However, it is a bit larger than those of STD and AEPA-modified devices. Similar to the inference from CA results, the contradiction can also be ascribed to the longer path for electron transport due to the longer molecular length of ABPA.
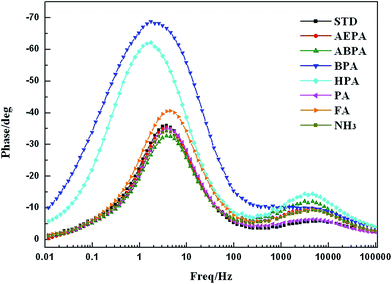
Compared to a Nyquist plot, a Bode plot can generally provide more information regarding the frequency response in a photoelectrochemical system. By plotting the relationship between phase and frequency, the Bode plots of the DSSCs with various SAM-modified TiO2 electrodes can be obtained, as displayed in Fig. 4. When TiO2 is modified by a different SAM, the electron transport rate at the TiO2/dye/electrolyte interface and corresponding electron lifetime (τe) would vary. τe can be calculated by the following equation:30
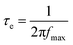 | (4) |
| fmax (Hz) | τe (ms) | |
|---|---|---|
| STD | 3.74 | 43 |
| AEPA | 3.74 | 43 |
| ABPA | 3.74 | 43 |
| BPA | 1.74 | 91 |
| HPA | 1.74 | 91 |
| PA | 4.54 | 35 |
| FA | 4.54 | 35 |
| NH3 | 3.74 | 43 |
To see the variation in the amount of dye adsorption on different SAM-modified TiO2 electrodes, the UV-vis absorption spectra of the dye-desorbed solutions obtained by immersing the SAM-modified photoanodes in 0.1 M of NaOH aqueous solutions are measured and compared. It has been observed that the dye-desorbed solution of the STD photoanode has the highest absorbance no matter in the UV or visible light region. This implies that the dye adsorption amount on the unmodified TiO2 electrode is the most. All the absorbances of other dye-desorbed solutions are lower. Although there is no big difference in the absorbances, the result has revealed that some sites on TiO2 surface originally bonded by dye molecules have been occupied by the small molecules of SAMs. The less dye adsorption of AEPA- and ABPA-modified DSSCs has also demonstrated that using amino-containing phosphonic acids to modify TiO2 electrodes is indeed beneficial to improve JSC and η.
Fig. 5 shows the photocurrent density–voltage (J–V) curves of the DSSCs with various SAM-modified TiO2 electrodes. The photovoltaic parameters are displayed in Table 3. It has been found that the JSC values show a trend similar to η, i.e. AEPA > STD or ABPA > PA or NH3 or FA > HPA > BPA. However, the magnitude of open-circuit voltage (VOC) seems to have no significant correlations with JSC and η. This indicates that the key factor to affect η is the variation of JSC. The DSSC with AEPA-modified electrode exhibits the largest JSC of 17.30 mA cm−2 and highest η of 6.67%, whereas those with BPA- and HPA-modified electrodes have the lowest η and JSC of 3.22 mA cm−2 and 4.14 mA cm−2, respectively. It can be seen from Tables 1 and 3 that even though the work functions of AEPA- and ABPA-modified electrodes are similar, the JSC of the DSSC with ABPA-modified electrode is a little smaller. This also can be attributed to the longer molecular length of ABPA than AEPA. Basically, the results have revealed that a larger JSC can be obtained when there is a lower work function. The magnitude of work function is the main basis for selecting molecules for surface modification. Meanwhile, molecular length should be taken into consideration.
| VOC (V) | JSC (mA cm−2) | FF | η (%) | |
|---|---|---|---|---|
| STD | 0.71 | 15.11 | 0.54 | 6.13 |
| AEPA | 0.72 | 17.30 | 0.54 | 6.67 |
| ABPA | 0.70 | 16.05 | 0.54 | 6.05 |
| BPA | 0.57 | 3.22 | 0.37 | 0.68 |
| HPA | 0.61 | 4.14 | 0.59 | 1.49 |
| PA | 0.71 | 14.6 | 0.57 | 5.91 |
| FA | 0.70 | 13.32 | 0.59 | 5.51 |
| NH3 | 0.71 | 14.13 | 0.562 | 5.56 |
The trend of JSC is also similar to that of CV and CA results. As revealed in Fig. 1, 2 and 5, the AEPA-modified electrode exhibits the largest redox current and the corresponding DSSC shows the largest JSC. As displayed in Table 3, the DSSCs with electrodes modified by PA, FA and NH3 show analogous photovoltaic characteristics. Another two modified by BPA and HPA exhibit the smallest JSC and lowest DSSC performance. All these are approximately consistent with the EIS results, namely, a smaller interfacial impedance favorable to charge transport and lower recombination probability results in a larger JSC. Compared to that with FA-modified electrode, the DSSC with PA-modified electrode exhibits both higher JSC and η. Apparently, the effect of surface modification on TiO2 by PA is superior to FA. This has verified the contention reported by Nilsing et al.26 Phosphonic acids are able to bind to TiO2 surface more strongly and stably than carboxylic acids.
4. Conclusions
Several molecules including phosphonic and carboxylic acids were selected to form SAMs for surface modification on TiO2 electrodes. The impacts of various SAMs on the photovoltaic characteristics of DSSCs were investigated. When the modification caused a lower work function and a larger redox current of an electrode, a smaller impedance at the TiO2/dye/electrolyte interface and thus a larger JSC were obtained. An exception was found in the ABPA-modified electrode due to the longer molecular length. When a longer molecule was used, the path for electron transport and the probability of charge recombination increased, and a smaller JSC was thereby resulted. Both photocurrent and dark current of a DSSC were suppressed when there was a larger interfacial impedance restraining electron transport at the interface. The AEPA-modified TiO2 electrode showed the largest redox current. The corresponding DSSC showed the smallest interfacial impedance. There were faster electron transport and reduced charge recombination in it. Accordingly, it exhibited the largest JSC of 17.30 mA cm−2 and highest η of 6.67%. Surface modification on TiO2 electrodes by amino-containing phosphonic acids could enhance DSSC performance efficiently. The magnitude of work function is the main basis for selecting molecules for surface modification. Molecular length should also be taken into consideration.Acknowledgements
Supports from the Ministry of Science and Technology Taiwan and National Chi Nan University are gratefully appreciated.References
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef.
- M. Grätzel, Acc. Chem. Res., 2009, 42, 1788 CrossRef PubMed.
- E. Ghadiri, N. Taghavinia, S. M. Zakeeruddin, M. Grätzel and J. E. Moser, Nano Lett., 2010, 10, 1632 CrossRef CAS PubMed.
- Z. Ji, G. Natu, Z. Huang, O. Kokhan, X. Zhang and Y. Wu, J. Phys. Chem. C, 2012, 116, 16854 CAS.
- W. Hong, Y. Xu, G. Lu, C. Li and G. Shi, Electrochem. Commun., 2008, 10, 1555 CrossRef CAS.
- V. Armel, M. Forsyth, D. R. MacFarlane and J. M. Pringle, Energy Environ. Sci., 2011, 4, 2234 CAS.
- K. Fan, T. Peng, J. Chen, X. Zhang and R. Li, J. Mater. Chem., 2012, 22, 16121 RSC.
- N. G. Park, J. V. d. Lagemaat and A. J. Frank, J. Phys. Chem. B, 2000, 104, 38 Search PubMed.
- S. Wu, H. Han, Q. Tai, J. Zhang, S. Xu, C. Zhou and Y. Yang, J. Power Sources, 2008, 182, 119 CrossRef CAS.
- S. M. Wang, W. W. Dong, R. H. Tao, Z. H. Deng, J. Z. Shao, L. H. Hu, J. Zhu and X. D. Fang, J. Power Sources, 2013, 235, 193 CrossRef CAS.
- R. G. Nuzzo and D. L. Allara, J. Am. Chem. Soc., 1983, 105, 4481 CrossRef CAS.
- G. Y. Choi, J. F. Kang, A. Ulman, W. Zurawsky and C. Fleischer, Langmuir, 1999, 15, 8783 CrossRef CAS.
- M. H. Keefe, R. V. Slone, J. T. Hupp, K. F. Czaplewski, R. Q. Snurr and C. L. Sterm, Langmuir, 2000, 16, 3964 CrossRef CAS.
- Y. Liu, R. C. Mills, J. M. Boncella and K. S. Schanze, Langmuir, 2001, 17, 7452 CrossRef CAS.
- A. B. Sieval, R. Linke, H. Zuilhof and E. J. R. Sudholter, Adv. Mater., 2000, 12, 1457 CrossRef CAS.
- M. P. Stewart and J. M. Buriak, Adv. Mater., 2000, 12, 859 CrossRef CAS.
- A. Abdelghani, S. Hleli and K. Cherif, Mater. Lett., 2002, 56, 1064 CrossRef CAS.
- T. Strother, W. Cai, X. Zhao, R. J. Hamers and L. M. Smith, J. Am. Chem. Soc., 2000, 122, 1205 CrossRef CAS.
- A. Ulman, Chem. Rev., 1996, 96, 1533 CrossRef CAS PubMed.
- C. Cater, M. Brumbach, C. Donley, R. D. Hreha, S. R. Marder, B. Domercq, S. H. Yoo, B. Kippelen and N. R. Armstrong, J. Phys. Chem. B, 2006, 110, 25191 CrossRef PubMed.
- A. Sharma, A. Haldi, W. J. Potscavage Jr, R. J. Hotchkiss, S. R. Marderb and B. Kippelen, J. Mater. Chem., 2009, 19, 5298 RSC.
- Y. T. Tao, G. D. Hietpas and D. L. Allara, J. Am. Chem. Soc., 1996, 118, 6724 CrossRef CAS.
- T. L. Breen, P. M. Fryer, R. W. Nunes and M. E. Rothwell, Langmuir, 2002, 18, 194 CrossRef CAS.
- I. Robel, V. Subramanian, M. Kuno and P. V. Kamat, J. Am. Chem. Soc., 2006, 128, 2385 CrossRef CAS PubMed.
- S. Pawsey, K. Yach and L. Reven, Langmuir, 2002, 18, 5205 CrossRef CAS.
- M. Nilsing, S. Lunell, P. Persson and L. Ojame, Surf. Sci., 2005, 582, 49 CrossRef CAS.
- C. P. Cho, C. C. Chu, W. T. Chen, T. C. Huang and Y. T. Tao, J. Mater. Chem., 2012, 22, 2915 RSC.
- C. P. Cho, H. Y. Wu and C. C. Lin, Electrochim. Acta, 2013, 107, 488 CrossRef CAS.
- D. Y. Kim and M. Kang, Mater. Chem. Phys., 2012, 136, 947 CrossRef CAS.
- Z. Gao, Z. Wu, X. Li, J. Chang, D. Wu, P. Ma, F. Xu, S. Gao and K. Jiang, CrystEngComm, 2013, 15, 3351 RSC.
| This journal is © The Royal Society of Chemistry 2016 |