Catalytic properties of nickel/sepiolite promoted with potassium and lanthanum in adiponitrile hydrogenation under mild conditions
Yang Lv,
Fang Hao*,
Shaofeng Xiong,
Pingle Liu* and
He'an Luo
College of Chemical Engineering, Xiangtan University, Xiangtan 411105, China. E-mail: liupingle@xtu.edu.cn; haofang.happy@163.com; Fax: +86 73158298172; Fax: +86 73158298267; Tel: +86 73158298005
First published on 8th June 2016
Abstract
Ni/sepiolite, potassium and (or) lanthanum doped Ni/sepiolite catalysts were prepared by the incipient impregnation method and characterized by N2 adsorption–desorption, temperature programmed reduction (TPR), hydrogen chemisorption, powder X-ray diffraction (XRD), ammonia temperature programmed desorption (NH3-TPD), transmission electron microscopy (TEM) and scanning electron microscopy (SEM). It was revealed that the potassium could inhibit the formation of the ACH by-product by neutralizing some acid sites on the catalyst, and the lanthanum could efficiently reduce the diameter and improve the dispersion of the active nickel particles. These catalysts were tested in liquid phase hydrogenation of adiponitrile (ADN). The products include 6-aminocapronitrile (ACN), hexamethylenediamine (HMDA), 1-azacycloheptane (ACH) and C12 compounds. It shows that the catalyst doped with potassium and lanthanum gives the best catalytic performance, the selectivity to ACN and HMDA reaches to 91.32% at 92.56% conversion of adiponitrile under 393 K and 2.0 MPa.
1 Introduction
ε-Caprolactam (CPL) is an important intermediate for the production of nylon-6 fibers and resins. The traditional technologies for ε-caprolactam production mainly use aromatic hydrocarbon as materials under multi-step processes and produce a large amount of ammonium sulfate by-product.1 Recently, researchers have paid much attention to new methods for ε-caprolactam production with lower cost and environmental pollution. Compared with the traditional technology, the route of butadiene hydrocyanation is one of green processes with many advantages. The process of butadiene hydrocyanation for ε-caprolactam production includes butadiene hydrocyanation to adiponitrile (ADN), the hydrogenation of adiponitrile to 6-aminocapronitrile (ACN) and the cyclization of 6-aminocapronitrile to ε-caprolactam in the presence of water.2–4 Among these steps, adiponitrile selective hydrogenation to 6-aminocapronitrile (ACN) is the key step, and its deep hydrogenation products include hexamethylenediamine (HMDA) and Schiff bases.4 6-Aminocapronitrile (ACN) and hexamethylenediamine (HMDA) are the intermediates for the manufacture of ε-caprolactam and nylon-66.Researchers have been concerned with gas phase and liquid phase hydrogenation of ADN.5–10 As to gas-phase ADN hydrogenation catalyzed by amorphous Ni–P or Ni–B, the main product is HMDA and the selectivity to ACN is only about 30%.11,12 Serra et al.5–7 reported the application of Ni–MgO catalyst in ADN hydrogenation, the ADN conversion is 87% and the ACN selectivity is 83%. Medina et al.13–19 investigated a series of Fe–Ni catalysts and alumina-supported nickel catalysts in gas-phase hydrogenation of ADN. Recently, RANEY® Ni or Co has been widely used in liquid phase hydrogenation of ADN under high pressure in ammonia or alkali metal hydroxide solution.20–25 However, besides the shortcomings of RANEY® catalyst, it needs a large amount of ammonia to inhibit the formation of secondary and tertiary amines. It has been reported that the selective hydrogenation of ADN to primary amine is structure sensitive and it is different to form active site of ACN, HMDA and 1-azacycloheptane (ACH). Therefore, the selectivity to ACN could be significantly improved by modifying the surface of the catalyst so as to reduce the generation active sites of HMDA and ACH. It has shown that the specific activity and selectivity of the nickel catalysts strongly depend on the promoters and the type of the supported metal.8,10,14,15,18,26 Sepiolite is a hydrous magnesium mineral and it has been used in adsorption and used as adsorbent for preconcentration of trace elements.27–32 It also could be used as wonderful support to load palladium, ruthenium and nickel to prepare hydrogenation catalysts.27–29,31
In this work, nickel/sepiolite (Ni/SEP), potassium and (or) lanthanum doped Ni/SEP catalysts were prepared by incipient impregnation method for the liquid phase hydrogenation of ADN to ACN and HMDA under mild conditions. The effects of potassium and lanthanum promoter on the catalytic performance were discussed.
2 Experimental
2.1 Materials
Adiponitrile (ADN) (98.5 wt%), 6-aminocapronitrile (ACN) (98 wt%) and hexamethylenediamine (HMDA) (99 wt%) were purchased from J&K scientific LTD. Sepiolite was purchased from Henan Xinglei Sepiolite Limited Company, (chemical composition: 42.21% SiO2, 20.57% MgO, 18.83% CaO, 0.37% Al2O3, 0.21% Fe2O3, 0.18% K2O, 0.08% Na2O, and 0.05% MnO). The analytical grade of hydrogen peroxide (30 wt%), Ni(NO3)2·6H2O, KNO3, La(NO3)3·6H2O and ethanol were purchased from Sinopharm Chemical Reagent Corporation Limited. H2 (99.99%) was purchased from Zhuzhou Diamond Gas Company.2.2 Catalyst preparation
Sepiolite was purified and micronized by a wet process. The catalysts were prepared by the incipient impregnation method. The pretreated sepiolite was firstly impregnated in the various combination of Ni(NO3)2·6H2O, La(NO3)3·6H2O and KNO3 solutions at 303 K for 10 h, the samples were dried at 393 K in air for 12 h and calcinated under flowing N2 at 623 K for 4 h and then reduced in a flowing H2 at 673 K for 4 h before cooling to ambient temperature in a flow of N2. The prepared catalysts were denoted as Ni/SEP, K–Ni/SEP, La–Ni/SEP and K–La–Ni/SEP, respectively. The nickel loading content of the catalysts is 20 wt%. And the content of the potassium and lanthanum promoter are 0.5 wt% and 2 wt%, respectively.2.3 Catalyst characterization
The specific surface area, pore volume and pore size distribution of the samples were obtained by nitrogen adsorption–desorption on a Quantachrome NOVA-2200e automated gas sorption system. Specific surface areas, meso-pore size distributions and micro-pore size distribution were calculated by Brunauer–Emmett–Teller (BET), Barrett–Joyner–Halenda (BJH) methods and Density-Functional-Theory (DFT) methods.Powder X-ray diffraction (XRD) patterns were determined under a D/max2500 TC diffract meter using Cu Kα radiation (λ = 1.542 Å). The tube voltage was 40 kV, the current was 30 mA, and the scan range was 2θ = 5–90° with a scanning rate of 1° min−1.
Temperature programmed reduction (TPR) was carried out in a Quantachrome ChemBET-3000 instrument, equipped with a 273 K to 1273 K procedure temperature-controlled furnace and a thermal conductivity detector (TCD). Each sample was firstly heated to 473 K in an argon flow for 2 h. Then, the sample was heated from 473 to 1173 K at a rate of 5 K min−1 under 40 mL min−1 5 vol% of H2/Ar flow.
Hydrogen chemisorption was measured on a ChemBET 3000 instrument equipped with a procedure temperature-controlled furnace and a thermal conductivity detector (TCD). The catalyst was previously reduced at 523 K for 12 h in hydrogen stream, and then the adsorbed hydrogen on the nickel surface was removed under a flowing argon stream. The catalyst was subsequently cooled to ambient temperature under the argon stream. The hydrogen pulses (0.02 mL) were injected until the eluted areas of consecutive pulses became constant. The amount of nickel atoms on the surface and nickel surface area were calculated by assuming the stoichiometric of one hydrogen molecule per two surface nickel atoms and the cross-sectional area of a nickel atom of 6.49 × 10−20 m2.
Ammonia temperature programmed desorption (NH3-TPD) was carried out in a Quantachrome ChemBET-3000 instrument equipped with a procedure temperature-controlled furnace and a thermal conductivity detector (TCD). The sample was firstly heated at 523 K in helium flow for 1 h. Then, it was heated at 353 K under 40 mL min−1 10 vol% of NH3/He flow for 30 min. The helium flow was used to purge the adsorbed NH3 until the eluted areas of consecutive pulses became constant. In the end, the ammonia temperature programmed desorption (NH3-TPD) was carried out in He flow from 353 to 1123 K at a rate of 10 K min−1.
The morphologies of the catalysts were observed with scanning electron microscope (SEM) on a JEOL JSM-6610LV scanning microscope operating at an accelerating voltage of 5 kV. The microstructures of the catalysts were observed by transmission electron microscopy (TEM) on a TecnaiG220 ST electron microscope working at less than 200 kV. The instrumental magnification ranged from 2 × 104 to 10 × 106. The samples were deposited on a copper grid and coated with a holey carbon film.
2.4 Procedure for the catalytic test
The liquid phase hydrogenation of ADN was carried out in a 100 mL Teflon-lined stainless steel autoclave equipped with temperature detector, pressure controller and magnetic stirrer. Generally, 1 g of catalyst, 50 mL of ethanol and 5 g of ADN were added into autoclave. The reactor was sealed and purged with H2 to exclude air, and then it was pressurized to 2 MPa with H2 under vigorous stirring when it was heated to the setting temperature. After reaction, the catalyst was separated by filtration, and then the contents of the reactants and the products were determined by GC (GC-14C, SHIMADZU) with a flame ionization detector (FID) and a 30 m DB-1701 capillary column using dimethyl phthalate (DMP) as the internal standard.3 Results and discussion
3.1 Characterization of catalysts
Table 1 lists BET surface areas of the samples. The surface area of sepiolite is larger than α-Al2O3, and it decreases when the metals are introduced into the sepiolite support. It has been reported that the La3+ can not diffuse into the micro pore channels of the needle-shaped silicate particles of the support because of its larger ion diameter.29 It suggests that there are some lanthanum oxides on the surface of the sepiolite, which lead to the decrement of the BET surface areas. It can be seen from Table 1 that lanthanum promoted catalysts such as La–Ni/SEP and K–La–Ni/SEP present relatively smaller BET surface areas.| Catalysts | BET surface area (m2 g−1) | Average pore diameter (nm) | Pore volume (cm3 g−1) |
|---|---|---|---|
| α-Al2O3 | 3.4 | 4.3 | 0.01 |
| SEP | 28.3 | 6.0 | 0.03 |
| K–Ni/α-Al2O3 | 9.3 | 13.1 | 0.03 |
| Ni/SEP | 17.3 | 13.2 | 0.06 |
| K–Ni/SEP | 11.5 | 15.0 | 0.06 |
| La–Ni/SEP | 5.9 | 23.1 | 0.02 |
| K–La–Ni/SEP | 8.6 | 21.6 | 0.06 |
Fig. 1(a) shows the nitrogen adsorption–desorption curves and BJH pore size distributions of different catalysts. All the isotherms are of type IV according to the IUPAC classification, representing capillary condensation of nitrogen within the meso-porous structure. All the hysteresis loops are of type H4, which is associated with slit-shaped pores resulting from cumulating of layer structures. According to the geometric effect and Kelvin equation, larger area of the hysteresis loop indicates more pores, which is in good agreement with the pore volume data in Table 1. The microporosity data of sepiolite, K–Ni/SEP and K–La–Ni/SEP are shown in Fig. 1(b). The micro-pore size distributions of these three typical samples were calculated by Density Functional Theory (DFT) methods. The result shows that sepiolite has micro- and meso-porous structure, while the micro-porous structure vanished after loading the active components, the reason may be that the micro-porous structures are blocked by the metal oxides during the catalysts preparation process.
 | ||
| Fig. 1 N2 adsorption–desorption isotherms and BJH pore size distributions of different samples and DFT pore size distribution of sepiolite (a), K–Ni/SEP (b), K–La–Ni/SEP (c). | ||
Fig. 2 show the SEM pictures of α-Al2O3, sepiolite and K–La–Ni/SEP. Fig. 2(a) and (b) show the cumulative layer of α-Al2O3 and typical fibrous layer morphology of sepiolite structure. The typical unit cell formula for sepiolite is Mg8Si12O30(OH)4(OH2)4·8H2O, and it is a Mg-rich clay mineral of trioctahedral type with a specific crystalline organization in channels.31,32 Fig. 2(c) and (d) illustrate an obvious supported structure of K–La–Ni/SEP. Moreover, it can be seen from Fig. 2(c) and (d) that the K–La–Ni/SEP possesses random porous structure and the metal oxides are covering on the surface of sepiolite, which lead to the decrement of the surface areas of the catalysts.
The TPR profiles of the catalysts before reduction by hydrogen are shown in Fig. 3. The TPR curves indicate that the reduction temperature is around 673 K and 850 K. The TPR peaks are significantly shifted toward lower temperature when lanthanum is introduced, it may be the electronic transfer due to the strong interaction between NiO and La2O3 and this promote the reduction of NiO.29 As the Ni metallic phase is easier formed from the reduction of Ni2+ ions in the lanthanum promoted catalysts, it indicates that the introduction of lanthanum is in favor of the ADN hydrogenation. The hydrogen consumption peak of NiO/SEP is wider than NiO–K2O/SEP, it shows that the reduction rate of NiO/SEP decreases, the reason may be that the interaction between K2O and NiO elevates the activation energy of the reduction of NiO and inhibit its reduction.26
Fig. 4 shows the NH3-TPD plots of the samples. It can be seen that all the samples only presents the weak acid sites desorption peak in the range of 530–625 K. Compared to sepiolite, the supported catalysts exhibit the weaker acidity. Moreover, it is apparent that K–Ni/SEP or K–La–Ni/SEP showed smaller acidic amounts than Ni/SEP, the reason may be that the introduction of potassium could neutralize some acid sites on the catalyst, which is in accordance with the reported that the modifying the catalysts with alkali metals could decrease the catalyst acidity.14,15,17,18,26 This characteristic of the supported SEP catalysts might influence the products distribution of the adiponitrile hydrogenation.
Fig. 5 shows the XRD patterns of the samples. The crystalline phases and crystallite sizes are depicted in Table 2. Compared with other catalysts, the diffraction peaks of nickel are broad and the diffraction peaks of nickel oxides are much sharper in K–Ni/SEP. Compared with La–Ni/SEP, K–La–Ni/SEP presents broad diffraction peaks of nickel and sharp diffraction peaks of nickel oxides. The results indicate that the introduction of potassium might inhibit the reduction of nickel oxides, which is well accord with the results of H2-TPR. Furthermore, the characteristic diffraction peaks of nickel in La–Ni/SEP and K–La–Ni/SEP are broader than those in Ni/SEP and K–Ni/SEP. And it can also be seen from Table 2 that the crystallite sizes of NiO and Ni decrease obviously when the lanthanum is added. It indicates that the decoration of lanthanum is helpful to the nickel dispersion, and the following results of hydrogen chemisorption and TEM also confirm this.
 | ||
| Fig. 5 XRD patterns of the samples: sepiolite (a), Ni/SEP (b), K–Ni/SEP (c), La–Ni/SEP (d), K–La–Ni/SEP (e). | ||
Fig. 6 shows the TEM images of different catalysts. As for Ni/SEP, the nickel nanoparticles agglomerate seriously. However, the nickel nanoparticles in K–Ni/SEP are more uniformly dispersed on the surface of the catalysts, the reason may be that the potassium effectively inhibits the excessive sintering of nickel particles during the reduction. Moreover, La–Ni/SEP and K–La–Ni/SEP exhibit more finely and uniformly nickel nanoparticles than K–Ni/SEP. The diameter of the nickel particles are approximately 20 nm in Ni/SEP and K–Ni/SEP while they are only 10 nm in La–Ni/SEP and K–La–Ni/SEP. Thus it can be seen from here that lanthanum can effectively reduce the diameter of nickel particles and improve the dispersion of the nickel particles.
The H2 chemisorption data are shown in Table 3. It can be seen that the amount of hydrogen chemisorption is remarkable increasing with the addition of potassium or lanthanum. K–La–Ni/SEP shows the best nickel dispersion, the largest hydrogen uptake quantity and metallic surface areas.
| Catalysts | H2 uptake quantity (μL g−1) | Metallic surface areas (m2 g−1) | Dispersion (%) |
|---|---|---|---|
| Ni/SEP | 14.2 | 2.9 | 0.31 |
| K–Ni/SEP | 25.1 | 3.8 | 0.62 |
| La–Ni/SEP | 22.3 | 3.4 | 0.79 |
| K–La–Ni/SEP | 30.1 | 4.6 | 0.86 |
3.2 Catalytic performance
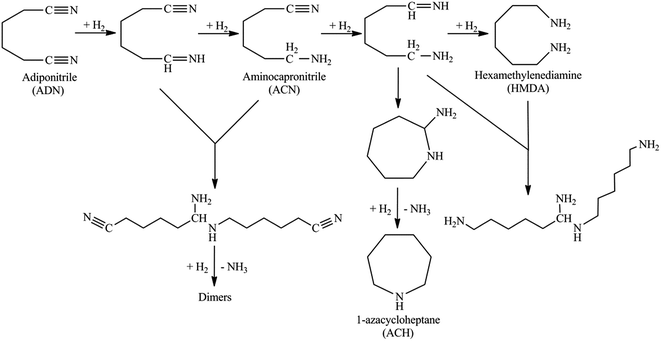
The products in adiponitrile (ADN) hydrogenation includes 6-aminocapronitrile (ACN), hexamethylenediamine (HMDA), 1-azacycloheptane (ACH) and C12 compounds.33 Numerous researchers have reported the reaction mechanism of the ADN hydrogenation.6,10,12,14 With the results presented in this work together with the previous studies, the products in ADN hydrogenation over SEP supported catalysts were proposed and shown in Scheme 1. In our previous work,26 crystalline Ni–K/α-Al2O3 were prepared and used in liquid phase hydrogenation of ADN, it exhibited good performance. However, the loading content of nickel is up to 30 wt% and the reaction temperature is as high as 423 K. Table 4 shows the results of the liquid phase hydrogenation of ADN catalyzed by sepiolite supported catalysts. It can be seen from Table 4 that Ni-based/sepiolite catalysts possess higher activity in ADN hydrogenation. The ADN conversion is up to around 99% under the reaction temperature of 413 K. The ADN conversion increases and the selectivity to ACN decrease obviously with the increment of the reaction temperature. Ni/SEP catalyst gives 65.58% selectivity to ACN and 18.55% selectivity to HMDA at the ADN conversion of 80.35%. However, the selectivity to ACN over the K–Ni/SEP catalyst reaches to 73.58% at almost the same ADN conversion under the reaction temperature of 393 K. The selectivity to ACN was greatly improved by doping potassium in sepiolite supported nickel catalysts, otherwise, the product of ACN would like to transform to ACH by-product. It has been demonstrated that the potassium, magnesium or other doping agents have a large impact on the activity and selectivity for nickel based catalysts in ADN hydrogenation.13–19 It is worth noting that the existence of potassium inhibits the well-known Thorpe–Ziegler intermolecular condensation of ADN to 2-aminomethylcyclopentylamine (C–C bond formation). It has been reported that the addition of a certain amount of an inorganic base in the reaction medium could improve the selectivity to aminonitrile in the hydrogenation of dinitriles over RANEY® Ni catalysts.2,20 The additives of inorganic base could form a stable heterocyclic compound and then interacted with ACN so as to impede its further hydrogenation to 1-amino-6-imino-hexane.| Catalyst | ADN conversion (%) | Temperature (K) | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| ACN | HMDA | ACH | ACN and HMDA | |||
| a Reaction conditions: stirring rate, 1000 rpm; PH2, 2.0 MPa; reaction time, 360 min; ethanol, 50 mL; and, 5 g; catalyst, 1 g. | ||||||
| Sepiolite | — | 393 | — | — | — | — |
| 80.35 | 393 | 65.58 | 18.55 | 15.87 | 84.13 | |
| Ni/SEPa | 83.19 | 403 | 53.64 | 25.54 | 20.82 | 79.18 |
| 96.15 | 413 | 24.38 | 35.29 | 40.33 | 59.67 | |
| 79.04 | 393 | 73.58 | 15.37 | 11.05 | 88.95 | |
| K–Ni/SEPa | 84.72 | 403 | 64.20 | 20.89 | 14.81 | 85.19 |
| 99.00 | 413 | 30.42 | 29.32 | 40.26 | 59.74 | |
| 84.31 | 393 | 64.20 | 16.13 | 19.67 | 80.33 | |
| La–Ni/SEPa | 95.90 | 403 | 45.49 | 23.36 | 31.15 | 68.85 |
| 99.45 | 413 | 0.56 | 45.37 | 54.07 | 45.93 | |
| 87.75 | 383 | 70.64 | 21.52 | 7.84 | 92.16 | |
| K–La–Ni/SEPa | 92.56 | 393 | 66.52 | 24.80 | 8.68 | 91.32 |
| 99.29 | 403 | 31.21 | 42.26 | 26.53 | 73.47 | |
Moreover, the potassium promoter could also neutralize some acid sites, and then inhibit the intermolecular condensation of 1-amino-6-imino-hexane to ACH by-product.14,15,17,18 It has been demonstrated that the neutralization of acid sites on the catalyst could indeed improve the hydrogenation selectivity.10 It can be seen from Table 4 that the conversion of ADN over La–Ni/SEP is obviously higher than that of Ni/SEP at the same reaction temperature. The lanthanum could not only improve the reduction of NiO, but also improve the dispersion of the nickel nanoparticles on the catalysts, as it can be seen from the TEM images of La–Ni/SEP and K–La–Ni/SEP. Among these Ni-based SEP catalysts, K–La–Ni/SEP gives the best catalytic performance of 66.52% selectivity to ACN at the ADN conversion of 92.56%. And the total selectivity to ACN and HMDA reaches to 91.32% under mild reaction conditions of 393 K and 2.0 MPa. Compared with K–Ni/α-Al2O3, the reaction temperature over K–La–Ni/SEP is reduced by 30 K.
3.3 The recycle of the catalysts
The recycle of K–La–Ni/SEP has been investigated and the results are listed in Table 5. After each reaction, K–La–Ni/SEP was separated by filtration, dried at room temperature and reduced at 673 K for 4 h. It can be seen from Table 5 that the catalytic activity decreases slightly in the second and third runs. The selectivity to ACN is above around 60%, and the total selectivity to ACN and HMDA is greater than 80%. However, the activity and the selectivity to ACN decrease obviously when the catalyst was used for the fourth times.| Run number | ADN conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| ACN | HMDA | ACH | ACN and HMDA | ||
| a Reaction conditions: stirring rate, 1000 rpm; PH2, 2.0 MPa; reaction time, 360 min; reaction temperature, 393 K; ethanol, 50 mL; and, 5 g; catalyst, 1 g. | |||||
| 1 | 92.36 | 66.50 | 24.85 | 8.65 | 91.35 |
| 2 | 86.35 | 62.14 | 27.60 | 10.26 | 89.74 |
| 3 | 84.26 | 60.36 | 25.74 | 18.90 | 81.10 |
| 4 | 76.32 | 54.67 | 22.69 | 22.64 | 77.36 |
4 Conclusions
Potassium and lanthanum promoted nickel based sepiolite catalysts were prepared and used in the ADN hydrogenation. The potassium could neutralize some acid sites on the catalyst and inhibit the formation of the ACH by-product. The lanthanum could efficiently reduce the diameter and improve the dispersion of the active nickel particles. Among these catalysts, K–La–Ni/SEP exhibits the best selectivity to ACN and HMDA at a higher ADN conversion. The selectivity to ACN and HMDA could reach to 91.32% at 92.56% conversion of ADN under mild conditions of 393 K and 2.0 MPa.Acknowledgements
This work was supported by NSFC (21276218, 21306160), Project of Hunan Provincial Education Department (14C1093, 14C1097) and Project of Hunan Provincial Science and Technology Department (2015GK1060).References
- H. Kajikuri, M. Kitamura and Y. Higashio, US Pat., 5304643, 1992.
- R. Fischer, P. Bassler, H. Luyken, F. Ohlbach, J. P. Merger, A. Ansmann, A. Rehfinger and G. Voit, US Pat., 6359178B1, 2002.
- P. Bassler, H. Luyken, G. Achhammer, T. Witzel, E. Fuchs, R. Fischer and W. Schnurr, US Pat., 5717090A, 1998.
- R. Sheldon, Green Chem., 2000, 2, G1–G4 RSC.
- M. Serra, P. Salagre, Y. Cesteros, F. Medina and J. E. Sueiras, Solid State Ionics, 2000, 134, 229–239 CrossRef CAS.
- M. Serra, J. Catal., 2002, 209, 202–209 CrossRef CAS.
- M. Serra, P. Salagre, Y. Cesteros, F. Medina and J. E. Sueiras, Appl. Catal., A, 2004, 272, 353–362 CrossRef CAS.
- L. Zhao, C. Y. Wang, J. X. Chen and J. Y. Zhang, Chin. J. Chem. Eng., 2007, 18, 685–688 CAS.
- S. Alini, A. Bottino, G. Capannelli, A. Comite and S. Paganelli, Appl. Catal., A, 2005, 292, 105–112 CrossRef CAS.
- D. Tichit, R. Durand, A. Rolland, B. Coq, J. Lopez and P. Marion, J. Catal., 2002, 211, 511–520 CrossRef CAS.
- X. B. Yu, H. X. Li and J. F. Deng, Appl. Catal., A, 2000, 199, 191–198 CrossRef CAS.
- H. X. Li, Y. P. Xu, H. Li and J. F. Deng, Appl. Catal., A, 2001, 216, 51–58 CrossRef CAS.
- F. Medina, P. Salagre, J. Sueiras and J. Fierro, J. Mol. Catal., 1991, 68, L17–L20 CrossRef CAS.
- F. Medina, P. Salagre, J. E. Sueiras and J. L. G. Fierro, J. Mol. Catal., 1993, 81, 387–395 CrossRef CAS.
- F. Medina, P. Salagre, J. E. Sueiras and J. L. G. Fierro, Solid State Ionics, 1993, 59, 205–210 CrossRef CAS.
- F. Medina, P. Salagre, J. E. Sueiras and J. L. G. Fierro, J. Chem. Soc., Faraday Trans., 1993, 89, 3507–3512 RSC.
- F. Medina, P. Salagre, J. E. Sueiras and J. L. G. Fierro, Appl. Catal., A, 1993, 99, 115–129 CrossRef CAS.
- F. Medina, P. Salagre, J. E. Sueiras and J. L. G. Fierro, J. Chem. Soc., Faraday Trans., 1993, 89, 3981–3986 RSC.
- F. Medina, P. Salagre, J. E. Sueiras and J. L. G. Fierro, J. Chem. Soc., Faraday Trans., 1994, 90, 1455–1459 RSC.
- M. C. Cotting, L. Gilbert and P. Leconte, US Pat., 5981790, 1999.
- S. B. Ziemecki, US Pat., 5151543, 1992.
- M. Harper, WO Pat., 0027526A1, 2000.
- R. P. Beatty and R. A. Paciello, US Pat., 5554778, 1996.
- R. P. Beatty and R. A. Paciello, US Pat., 5559262, 1996.
- R. P. Beatty and R. A. Paciello, US Pat., 5599962, 1997.
- H. G. Liao, S. H. Liu, F. Hao, P. L. Liu, K. Y. You, D. D. Liu and H. a. Luo, React. Kinet., Mech. Catal., 2013, 109, 475–488 CrossRef CAS.
- J. A. Anderson, S. E. Falconer and M. Galán-Fereres, Spectrochim. Acta, Part A, 1997, 53, 2627–2639 CrossRef.
- F. M. Bautista, J. M. Campelo, A. Garcia, R. Guardeño, D. Luna and J. M. Marinas, J. Mol. Catal. A: Chem., 1996, 104, 229–235 CrossRef CAS.
- S. Damyanova, L. Daza and J. L. G. Fierro, J. Catal., 1996, 159, 150–161 CrossRef CAS.
- S. Liu, M. Chen, L. Chu, Z. Yang, C. Zhu, J. Wang and M. Chen, Int. J. Hydrogen Energy, 2013, 38, 3948–3955 CrossRef CAS.
- Y. Ma and G. Zhang, Chem. Eng. J., 2016, 288, 70–78 CrossRef CAS.
- K. Núñez, R. Gallego, J. M. Pastor and J. C. Merino, Appl. Clay Sci., 2014, 101, 73–81 CrossRef.
- F. Mares, J. E. Galle, S. E. Diamond and F. J. Regina, J. Catal., 1988, 112, 145–156 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |