Synthesis and catalytic application of N-heterocyclic carbene copper complex functionalized conjugated microporous polymer†
Abstract
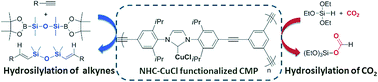
A N-heterocyclic carbene copper(I) complex functionalized conjugated microporous polymer (CMP-NHC-CuCl) was synthesized by palladium-catalyzed Sonogashira cross-coupling chemistry. The resulting CMP-NHC-CuCl proved to be a good heterogeneous catalyst in the hydrosilylation of functionalized terminal alkynes with boryldisiloxane to afford (β,β)-(E)-vinyldisiloxane with high stereoselectivity, and the catalyst could be used four times without obvious loss in catalytic activity. Moreover, CMP-NHC-CuCl was also efficient in catalyzing the hydrosilylation of CO2 with triethoxysilane to form silyl formate under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...