Micro-fibrillated cellulose reinforced eco-friendly polymeric resin from non-edible ‘Jatropha curcas’ seed waste after biodiesel production†
Muhammad M. Rahman and
Anil N. Netravali*
Department of Fiber Science & Apparel Design, Cornell University, 37 Forest Home Drive, Ithaca, New York 14853, USA. E-mail: ann2@cornell.edu; Tel: +1 607 255 1875
First published on 26th April 2016
Abstract
Eco-friendly polymeric resin with desirable mechanical and physical properties was developed from non-edible protein extracted from ‘Jatropha curcas’ (Jatropha) seed cake, so far considered as an agro-waste after oil extraction for bio-diesel conversion. A green, facile and cost-effective water-based casting and evaporation method was applied to fabricate Jatropha Protein (JP) based resin sheets. High molecular weight and the presence of reactive amino acids (a high content of arginine) in JP provide the basis to form a sustainable polymeric material. Also, JP resins were found to display diverse mechanical properties ranging from brittle and rigid to ductile and soft depending on the external modifiers such as plasticizer, cross-linker and reinforcing element used. Experimental studies using 10% sorbitol as a plasticizer, 10% glyoxal as a cross-linker and 20% microfibrillated cellulose (MFC) as a reinforcing element rendered JP resin with promising mechanical, thermal and physico-chemical properties. A favorable comparison between the modified JP and various polymers opens up possibilities for a sustainable alternative from non-edible protein-based agro-wastes that can reduce the dependency on biobased polymers from edible sources and petroleum based non-degradable polymers for applications such as fiber reinforced composites.
1. Introduction
Polymers derived from unsustainable fossil resources have been most widely used in a broad variety of applications ranging from simple packaging to complex structural components. In the past few decades their use has exploded in all parts of our lives because of the ability to tailor their properties to the application. The most noticeable negative impact of this fossil resource utilization, however, has been the generation of unmanageable amounts of waste created by their disposal.1 Unfortunately, these petroleum based polymer wastes do not biodegrade even after several decades under normal environmental conditions.2 They may, however, break down into toxic micro-fragments over a long period that are hazardous to the environment and ecological systems.3,4 Increased concerns associated with the deteriorating environment, waste disposal difficulties and costs, and the alarming rate of fossil resource depletion have fueled a growing interest in developing biodegradable and eco-friendly materials from renewable resources with similar performances.5–7 Among the myriad of renewable resources, plant proteins have attracted considerable attention as a new class of sustainable and biodegradable polymer due to their excellent properties.8–12 They offer excellent functional properties through the formation of numerous inter and intra-molecular bonds due to the presence of various polar amino acids.13 However, thus far, research has been mainly focused on commercially available edible plant proteins such as soy and wheat and starches such as corn, potato, etc., to produce ‘green’ materials.2,8,11,14–16 Such materials have raised ethical questions on utilizing food sources for technical applications.2 Considering the increased demand for edible proteins and starches for the growing world population, it would be advantageous to explore non-edible protein sources for various technical applications so as to resolve the issue of food security.Over the past decade, non-edible energy crop ‘Jatropha curcas’ or commonly known as ‘Jatropha’, has received significant attention as one of the best candidates for biodiesel production due to its high oil content in the range of 50–60%.17,18 Also, the wide adaptability of Jatropha to grow under most climatic conditions makes it very promising over other energy crops such as castor, linseed or karanja.17 At the same time, Jatropha seed kernel is rich in non-edible protein (27–32%) while kernel residue after oil extraction (seed cake) has about 60% protein content.19 However, this defatted seed cake remains as waste or low-value stream due to the presence of phytotoxins and anti-nutritional compounds such as phorbol esters, curcin, trypsin inhibitor, lectin and phytate.20
Interestingly, the Jatropha seed cake has higher protein content in comparison to the commercial defatted soy flour (50–54%) which has been the most widely used protein-based biopolymer, from edible sources, for ‘green’ packaging and fiber reinforced composites. A comparable amino acid profile of Jatropha Protein (JP) with that of soy protein21 suggests both to have similar chemical reactivity. Moreover, the relatively higher molecular weight of JP could be beneficial in obtaining higher functional properties as compared to soy protein.21 Another inherent advantage of JP over soy or other edible proteins is the presence of the toxins that could provide the necessary resistance against microbial and fungi attack without any additives. The non-edible Jatropha protein can be beneficially utilized over the edible ones (e.g. soy protein) as an alternative ‘green’ polymer or resin for non-food green composite applications such as casing, packaging, furniture/cabinetry/shelving, automotive panels, and other indoor structures in housing and transportation.
In the present study, the possibilities of JP as a biobased ‘green’ resin in terms of mechanical, thermal and physicochemical properties for packaging and composite applications have been addressed. As an initial study, the functional properties of pure JP as resin for use in composites were improved stepwise using an environment-friendly plasticizer ‘sorbitol’, a non-toxic renewable cross-linker ‘glyoxal’ and a plant-derived reinforcing agent ‘microfibrillated cellulose’ (MFC) through a facile, cost-effective and ‘green’ fabrication process. The functional properties of the modified JP resin showed characteristics comparable with many biobased edible polymers utilized in fully green composite materials so far.
2. Experimental
2.1 Materials
‘Jatropha curcas’ seed press cake (JSC) was purchased from Global Clean Energy Holdings, Inc., Torrance, CA, USA. Analytical grade sodium hydroxide (NaOH) pellets, hydrochloric acid (37%), ammonium nitrate (NH4NO3, ≥98% purity), reagent grade D-sorbitol (≥98% purity), glyoxal (≥40% in water) and copper(II) sulfate pentahydrate (CuSO4·5H2O, ≥98% purity) were purchased from Sigma-Aldrich Chemical Co., Allentown, PA, USA. Microfibrillated cellulose (MFC, Celish KY100G, water slurry containing fiber content of approximately 10 wt%) was obtained from Daicel Chemical Industries Ltd., Japan. Milli-Q deionized (DI) water (resistivity, 18.2 MΩ cm, Millipore RiOs and Elix water purification systems, Millipore Corporation, MA) was used in all experiments in this study.2.2 Processing of JP powder
As-obtained JSC was ground into powder using an analytical mill (Model: UX 04305, Cole-Parmer, IL, USA) and passed through 300 μm mesh sieve to obtain homogenous micro particles. The sieved powder was then suspended into DI water at a solid–liquid weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and adjusted to pH of 11.5 ± 0.2 using 1.0 N NaOH. The suspension was stirred at 400 rpm at room temperature (RT) for 1 h. The insoluble residue from the suspension was discarded by centrifugation at 10
10 and adjusted to pH of 11.5 ± 0.2 using 1.0 N NaOH. The suspension was stirred at 400 rpm at room temperature (RT) for 1 h. The insoluble residue from the suspension was discarded by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, and the supernatant was collected using vacuum filtration on a Whatman® filter paper (Grade 42, retention: 2.5 μm). The pH of the supernatant was adjusted to 5.0 ± 0.2 using 1 N HCl and stirred mechanically at 400 rpm for 20 min for isoelectric precipitation of JP. The suspension was kept static at 4 °C for 2 h in a refrigerator. The resulting brown precipitation was separated by centrifugation at 10
000 rpm for 10 min, and the supernatant was collected using vacuum filtration on a Whatman® filter paper (Grade 42, retention: 2.5 μm). The pH of the supernatant was adjusted to 5.0 ± 0.2 using 1 N HCl and stirred mechanically at 400 rpm for 20 min for isoelectric precipitation of JP. The suspension was kept static at 4 °C for 2 h in a refrigerator. The resulting brown precipitation was separated by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and washed using 50–50 ethanol–water mixture. This washing procedure was repeated three times and recollected by centrifugation. The washed protein was then lyophilized, milled and stored at RT before characterizing their properties. It is referred to as ‘JP powder’ in this paper.
000 rpm for 10 min and washed using 50–50 ethanol–water mixture. This washing procedure was repeated three times and recollected by centrifugation. The washed protein was then lyophilized, milled and stored at RT before characterizing their properties. It is referred to as ‘JP powder’ in this paper.
2.3 Preparation of JP resins
JP resins in sheet form were prepared using solution casting and solvent evaporation to characterize their properties. At first, JP powder was homogenized in DI water at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v) by a magnetic stirrer for 15 min. Sorbitol, as plasticizer, at 10 wt% of JP powder was added and stirred further for 15 min. After stirring, the pH of the solution was adjusted to 11.5 ± 0.2 using a 1 N NaOH solution and was stirred for 20 min at 80 °C. This pre-cured JP resin solution was then dried on Teflon® coated glass plates at RT overnight followed by a 4 h drying in an air circulating oven maintained at 70 °C. The dried plasticized JP resin was finally hot-pressed using a hydraulic press (Carver Inc., Wabash, IN) at 130 °C for 10 min under a pressure of 8.5 MPa to complete the curing. Pure JP in the sheet form was prepared without sorbitol using the same process.
10 (w/v) by a magnetic stirrer for 15 min. Sorbitol, as plasticizer, at 10 wt% of JP powder was added and stirred further for 15 min. After stirring, the pH of the solution was adjusted to 11.5 ± 0.2 using a 1 N NaOH solution and was stirred for 20 min at 80 °C. This pre-cured JP resin solution was then dried on Teflon® coated glass plates at RT overnight followed by a 4 h drying in an air circulating oven maintained at 70 °C. The dried plasticized JP resin was finally hot-pressed using a hydraulic press (Carver Inc., Wabash, IN) at 130 °C for 10 min under a pressure of 8.5 MPa to complete the curing. Pure JP in the sheet form was prepared without sorbitol using the same process.
Glyoxal at 10 wt% of JP powder was added to plasticized JP resin solution as a cross-linker after 20 min of pre-curing at 80 °C. The solution was stirred for 10 min at the same temperature. The pre-cured cross-linked JP was dried and cured using the same cycle as described above to get it in the sheet form. The JP resin without any modifiers is termed as ‘Pure JP’ resin. The JP resin with sorbitol is termed as ‘Plasticized JP’ resin and plasticized JP resin with glyoxal is termed as ‘Cross-linked JP’ resin.
2.4 Preparation of MFC–reinforced JP resin
Preparation of MFC–reinforced JP resin in the sheet form was accomplished as follows: as-received MFC was added to DI water at a solid–liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 and dispersed using a VWR 250 homogenizer (VWR International, PA, USA) for 15 min at 20
150 and dispersed using a VWR 250 homogenizer (VWR International, PA, USA) for 15 min at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm followed by overnight high-speed magnetic stirring. The JP powder was added to the MFC suspension at a ratio of 80
000 rpm followed by overnight high-speed magnetic stirring. The JP powder was added to the MFC suspension at a ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/w). Sorbitol at 10 wt% of JP was also added at this stage. The plasticized JP–MFC resin solution was stirred for 30 min and then adjusted to pH of 11.5 ± 0.2. The alkaline mixture was stirred further for 30 min at 80 °C. Glyoxal at 10 wt% of JP powder was added to the mixture after 30 min and stirring was continued for 20 min at the same temperature. The pre-cured resin solution was dried at 70 °C in an air circulating oven for 6 h followed by overnight drying at RT. The dried resin sheets were cured using the same cycle as described in Section 2.3. Cross-linked JP resin with 20 wt% MFC is termed as ‘MFC–reinforced JP’ resin. All resin sheets were conditioned at 21 ± 1 °C and 65 ± 2% RH for 24 h using a saturated ammonium nitrate (NH4NO3) solution.22
20 (w/w). Sorbitol at 10 wt% of JP was also added at this stage. The plasticized JP–MFC resin solution was stirred for 30 min and then adjusted to pH of 11.5 ± 0.2. The alkaline mixture was stirred further for 30 min at 80 °C. Glyoxal at 10 wt% of JP powder was added to the mixture after 30 min and stirring was continued for 20 min at the same temperature. The pre-cured resin solution was dried at 70 °C in an air circulating oven for 6 h followed by overnight drying at RT. The dried resin sheets were cured using the same cycle as described in Section 2.3. Cross-linked JP resin with 20 wt% MFC is termed as ‘MFC–reinforced JP’ resin. All resin sheets were conditioned at 21 ± 1 °C and 65 ± 2% RH for 24 h using a saturated ammonium nitrate (NH4NO3) solution.22
2.5 Characterization
Proximate chemical compositions of JSC and JP powder were determined according to standard ‘Association of Analytical Communities (AOAC)’ methods. The protein content was obtained by Leco FP-528 Nitrogen/Protein analyzer (Leco Corporation, St. Joseph, MI) using a conversion factor of 6.25. All analyses were conducted by Dairy One, Ithaca, NY, in triplicate, to confirm the reproducibility.CIELAB color parameters, L*, a*, and b* of all resin sheet specimens were measured using Macbeth Color-eye spectrophotometer (Model: M2020PT, Newburgh, NY) to calculate whiteness index (WI). The WI of the specimens was calculated using the equation:
Moisture content (MC) and water solubility (WS) of all resin sheet specimens (∼10 × 10 mm) were measured according to the methods described by Rhim et al.23 and Cuq et al.,24 respectively. Also, the specimens (∼10 × 10 mm) were immersed in 30 mL DI water and placed on a platform shaker (Innova™ 2300, New Brunswick Scientific Inc., New Brunswick, NJ) at 80 °C and 200 rpm for 24 h to observe, visually, the swelling and/or the disintegration behavior of the sheets. Photographic images were taken after shaking for 24 h. Water absorption (WA) values were measured for specimens (∼10 × 10 mm) according to ASTM D570-98 standard. The specimens were dried at 110 °C for 24 h and weighed to obtain dry weight (W0). The specimens were then conditioned at 98% RH and 21 °C in a desiccator using a saturated salt solution of CuSO4·5H2O.25 The specimens were removed from the desiccator at every 2 h intervals and reweighed (Wt) until equilibrium value was reached. WA value at any time was calculated using the equation:  . Three specimens of each composition were tested and the mean value is reported.
. Three specimens of each composition were tested and the mean value is reported.
Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectra were recorded in the range of 4000–800 cm−1 wave-numbers using a Nicolet Magna 560 spectrometer (Nicolet Instrument Corporation, Madison, WI) with a split pea accessory for ATR. Each spectrum was an average of 64 scans with a resolution of 4 cm−1.
Thermogravimetric analysis (TGA) of all resin sheet specimens was conducted using a TGA-2050 instrument (TA Instruments, Inc., New Castle, DE) to characterize their thermal stability and degradation behavior. Specimens weighing 3–5 mg were scanned from 30 to 600 °C at a heating rate of 10 °C min−1 under a flow of 60 mL min−1 nitrogen gas. The initial degradation temperature was determined using Universal Analysis software (TA Instruments). In addition, long-term thermal stability test of specimens weighing 6–7 mg was conducted by isothermal TGA experiment. The specimens were heated rapidly to the experiment temperature at 60 °C and maintained at this temperature for a period of 30 h. Differential scanning calorimetry (DSC) was performed using DSC 2920 thermal analyzer (TA Instruments, Inc., New Castle, DE) under a flow of 60 mL min−1 nitrogen gas to characterize heat-induced denaturation behavior of resin sheet specimens. The specimens (approximately 5 mg) were first heated from 30 to 110 °C at a rate of 10 °C min−1 to remove any thermal history, held at that temperature for 1 min, then cooled to 30 °C at a cooling rate of 20 °C min−1 before carrying out the second heating scan to 220 °C at a heating rate of 10 °C min−1. The denaturation temperature (Tden) was determined from the second heating scans using Universal Analysis software. Tden was considered as the maximum peak temperature of the endothermic phenomenon of the specimens during heating scans. Four specimens of each composition were investigated to ensure repeatability in TGA and DSC.
Mechanical properties of the sheet specimens in both dry and conditioned states were determined according to ASTM D638 with a universal tensile testing machine (Instron 5566, Canton, MA). The dry state refers to the sheet specimens without conditioning while the conditioned state refers to conditioning of the specimens as mentioned in Section 2.4. Sheets with dimensions of 50 × 5 mm were tested at a strain rate of 1% min−1 and a gauge length of 30 mm. Six specimens of each composition were tested to report the average values with standard deviations. Specimen surfaces and fracture surfaces after tensile tests were analyzed using a LEO 1550 field emission scanning electron microscope (FESEM, LEO Electron Microscopy, Cambridge, UK) at 3 kV accelerating voltage. All of the statistical analyses were performed using JMP statistical software (SAS Institute, NC).
3. Results and discussion
3.1 Proximate chemical composition of JSC and JP powder
Proximate chemical compositions of as-received JSC and extracted JP powder are presented in Table 1. Compositions of commercial soy protein concentrate (SPC) and soy protein isolate (SPI) are also presented in Table 1 for comparison. The protein content in JSC was about 23–25% with high content of fat (∼10%) and crude fiber (∼35%). Fat content mainly depends on the oil extraction method. Usually, solvent extraction is a much more efficient method compared to screw-pressing in terms of fat extraction from seeds.26 The JSC, used in this study, was defatted by screw-pressing of the seed for which higher fat contents can be expected. However, the presence of fat (∼10%) is advantageous in the present application as it acts as a hydrophobic plasticizer and reduces the need for external plasticizer. A significantly high fiber content (∼35%) was obtained in JSC as the whole seed, with the shell, was screw-pressed. It is clear that protein content, after removal of crude fibers from JSC, would be higher than that of commercial soy flour (∼52%) that is extracted from de-hulled seedcake. In the present study, the extracted JP powder after lab processing contained about 81–83% protein which is between the protein contents seen for SPC and SPI. The protein extraction yield was 0.17 ± 0.02 g JP per g of JSC.| Constituent | JSC | JP | SPC | SPI |
|---|---|---|---|---|
| Crude protein (%) | 23.1–25.3 | 81.0–83.2 | 68.0–72.0 | 90.0–92.0 |
| Crude fat (%) | 9.8–10.8 | 9.1–10.1 | 1.0–1.5 | 0.5–0.7 |
| Crude fiber (%) | 34.2–36.3 | — | 4.5–5.5 | 0.1 |
| Carbohydrates (%) | 4.2–4.8 | 0.4–0.8 | 17.5–20.0 | 0.3–0.5 |
| Ash (%) | 5.9–6.6 | 1.0–1.1 | 5.0–6.0 | 4.5–5.0 |
3.2 Properties of pure JP resin
Table 2 presents average thickness, density and whiteness index of pure and modified JP resin sheets. Sheets made of pure JP resin had smooth and homogeneous surface characteristics. They had a thickness and density of 0.25 ± 0.01 mm and 1.09 ± 0.01 g cm−3, respectively (Table 2). The color of the sheets was light brown with an average whiteness index (WI) of 55.7. Color and transparency are not considered to be important issues as the major application of JP would be used as resin in fiber-reinforced composites for structural and other applications. However, change in color after the Maillard reaction with glyoxal can be used to confirm the protein crosslinking.| Specimens | Thickness (mm) | Density (g cm−3) | Whiteness index (%) |
|---|---|---|---|
| Pure JP | 0.25 ± 0.01 | 1.09 ± 0.01 | 55.7 |
| Plasticized JP | 0.26 ± 0.01 | 1.16 ± 0.03 | 56.0 |
| Cross-linked JP | 0.32 ± 0.01 | 1.29 ± 0.01 | 54.0 |
| MFC–reinforced JP | 0.31 ± 0.01 | 1.33 ± 0.02 | 54.9 |
Table 3 presents the denaturation temperatures (Tden) and initial degradation (Td) temperatures of pure and modified JP resins. The heat denaturation and initial degradation temperatures of the pure JP resin were observed at 155.3 ± 2.0 °C and 245.5 ± 2.2 °C, respectively. Table 4 presents the tensile properties of various JP resins in dry state along with corresponding moisture content (MC) values. Young's modulus and tensile strength (fracture stress) of the pure JP resin sheets were found to be 2.12 GPa and 22.1 MPa, respectively, in their dry state. However, fracture strain of pure JP resin was very low, about 1%, indicating its highly brittle nature. Properties of modified resins are discussed later in Section 3.3.
| Specimen sheets | Denaturation temperature (Tden) (°C) | Initial degradation temperature (Td) (°C) |
|---|---|---|
| a Values in parentheses are % coefficient of variation. | ||
| Pure JP | 155.3 (1.4)a | 245.4 (1.1)a |
| Plasticized JP | 148.6 (1.2) | 229.0 (1.0) |
| Cross-linked JP | 164.6 (1.2) | 246.0 (0.9) |
| MFC–reinforced JP | 157.0 (1.2) | 252.3 (1.0) |
| Specimen sheets | Young's modulus (MPa) | Tensile strength (MPa) | Fracture strain (%) | Moisture content (%) |
|---|---|---|---|---|
| a Values in parentheses are % coefficient of variation. | ||||
| Pure JP | 2120 (9.3)a | 22.1 (11.4)a | 1.0 (30.0)a | 3.6 (5.6)a |
| Plasticized JP | 1390 (6.2) | 16.8 (8.9) | 3.2 (28.1) | 3.9 (7.7) |
| Cross-linked JP | 1546 (6.4) | 19.9 (10.5) | 2.7 (25.9) | 3.3 (6.0) |
| MFC–reinforced JP | 2272 (6.1) | 29.3 (9.6) | 2.3 (39.1) | 3.2 (6.3) |
Another critical property of proteins is their interaction with water, particularly the water absorption, and the resulting changes in their properties. In the present case, pure JP resin sheet became softer when initially placed in water. However, after 24 h of immersion, both specimen dimensions (length and width) increased significantly as a result of water absorption. The specimens also showed slight warping. This phenomenon is shown in Fig. 1(a) inset photograph. However, the sheets did not break or disintegrate in water. Even, vigorous shaking (∼200 rpm) at high temperature (∼80 °C) for 24 h could not break the specimens into pieces or disintegrate. Fig. 2 shows the photographs of pure and modified JP resin sheets after shaking at 200 rpm in 80 °C in water for 24 h. Earlier research involving soy protein had shown that the specimens completely disintegrate in water after being continuously shaken (∼100 rpm) for three days at 80 °C.27 Also, in our preliminary experiments, complete disintegration of SPI resin sheets was observed in less than 24 h at 80 °C and 200 rpm.
 | ||
| Fig. 2 Photographs of pure and modified JP resin sheets after shaking at 200 rpm in 80 °C in water for 24 h. | ||
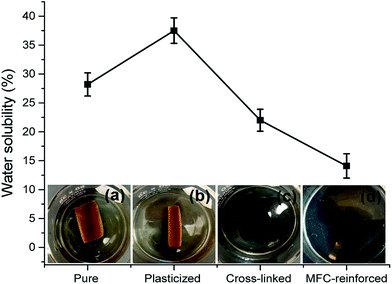
From the water solubility test as shown in Fig. 1 inset photographs, pure JP resin was found to have better water stability with significant gel fraction (approximately 72%) in comparison to the resin based on soy protein.27 Even, the gel fraction was higher than that of cross-linked soy protein.27 Higher cystine residue content in JP that results in higher number of intra- and inter-molecular disulfide linkages might be responsible for the JP protein to be less soluble.28 This phenomenon can also be attributed to several other factors including high fat content, higher molecular weights, the specific amino acid composition and sequences of JP.26,29 Better water stability is an added advantage of JP over soy protein in ‘green’ composite applications. Other specimens shown in Fig. 1 and 2 have been discussed later in Section 3.3.3.
3.3 Properties of modified JP resin
Pure JP resin was too brittle to be used as resin in composites and can be toughened by adding a plasticizer in the sheet-forming solution. Plasticizers are known to reduce protein–protein interactions by replacing protein–plasticizer hydrogen interactions as well as to increase the free volume of the protein systems.30,31 As a result, the flexibility and mobility of the protein chains increase, in turn, reducing the brittleness of the JP resin. Sorbitol, proven to be a suitable plasticizer for protein in earlier studies, was used at 10 wt% of JP powder in this study.28,32 However, it makes the protein moisture sensitive in humid conditions due to the hydrophilic nature of sorbitol. Hence, a dialdehyde based fully biodegradable and renewable cross-linker ‘glyoxal’ was used to increase water resistance and mechanical properties of the JP–plasticizer network system as a next step. In previous studies, glyoxal has been found to be an effective cross-linker for other plant-based proteins with higher content of arginine and lysine in their amino acid profiles.32,33 The key cross-linking reaction occurs between aldehyde groups of glyoxal and amine groups of lysine as well as guanidyl groups of arginine in JP resin, at alkaline pH.34 It is to be noted that arginine content (∼14 g per 16 g total nitrogen)20 in JP was observed to be significantly higher in comparison to some other proteins including soy (∼6 g per 16 g total nitrogen) and wheat (∼2.4 g per 16 g total nitrogen).21,35As an additional step to further enhance the mechanical properties of the cross-linked JP resin, MFC was homogeneously dispersed in it. MFC can be easily processed in water and has high affinity to protein molecules because of their polar groups.36 Further, it is an inherently connected web-like network with polar hydroxyl groups and exceptionally high specific strength and stiffness37 which provide the basis to select MFC as potential reinforcement of cross-linked JP resin. MFC at 20 wt% in cross-linked JP resin was incorporated in this study as a threshold after which agglomerations and uneven surfaces in the resin sheets were observed.
The change in the whiteness index, i.e., color, was not significant after plasticizing JP resin in comparison to pure JP resin (p-value > 0.05). However, cross-linking changed from the light brown color of the JP resin to dark brown color (p-value < 0.05). The Maillard reaction is responsible for the dark color of the cross-linked JP resin.27 The reaction takes place between the amine groups of protein and the aldehyde groups of glyoxal in the presence of heat and produces irreversible adducts on proteins both intra- and inter-molecularly, collectively known as advanced glycation end (AGE) products which are identified by the color change.32 A slight increase in the whiteness index of MFC–reinforced resin can be attributed to the white color of MFC itself (p-value > 0.05).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), amide II (N–H bending), and amide III (C–N and N–H stretching) linkages, respectively.33 The peaks around 2928 and 2854 cm−1 are related to the symmetric and asymmetric C–H stretching of CH2 and CH3 groups and the peak at 1455 cm−1 is related to the C–H bending in JP. A peak around 1745 cm−1 is associated with C
O stretching), amide II (N–H bending), and amide III (C–N and N–H stretching) linkages, respectively.33 The peaks around 2928 and 2854 cm−1 are related to the symmetric and asymmetric C–H stretching of CH2 and CH3 groups and the peak at 1455 cm−1 is related to the C–H bending in JP. A peak around 1745 cm−1 is associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption due to the presence of fat in JP. In plasticized JP resin, the peak around 1155 cm−1 associated with C–O–H groups in pure JP has shifted at 1161 cm−1 and the intensity has been increased significantly due to the possible interaction between plasticizer (OH group of sorbitol) and protein.28
O absorption due to the presence of fat in JP. In plasticized JP resin, the peak around 1155 cm−1 associated with C–O–H groups in pure JP has shifted at 1161 cm−1 and the intensity has been increased significantly due to the possible interaction between plasticizer (OH group of sorbitol) and protein.28
After cross-linking, the amide-I band shifted to a higher wave number, from 1625 cm−1 (plasticized JP) to 1635 cm−1 (cross-linked JP), suggesting decreased hydrogen bonds in the system.38 Also, the intensity of the peak at 1745 cm−1 is significantly reduced. The reaction between glyoxal and JP might reduce the density of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups on the surface of the sheet.9 However, a clear confirmation of the cross-linking reactions through ATR-FTIR is difficult due to the spectral complexity of the protein. Particularly, evidence of methylene bridge formation due to cross-linking is not obvious since characteristic CH2 bands are already present in the protein itself.12 In MFC–reinforced JP resin, the intensity of the band at 1034 cm−1 for C
O groups on the surface of the sheet.9 However, a clear confirmation of the cross-linking reactions through ATR-FTIR is difficult due to the spectral complexity of the protein. Particularly, evidence of methylene bridge formation due to cross-linking is not obvious since characteristic CH2 bands are already present in the protein itself.12 In MFC–reinforced JP resin, the intensity of the band at 1034 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching increased due to the presence of MFC.39 Also, the peak at 1080 cm−1 observed in the cross-linked JP for C–OH group shifted significantly to 1055 cm−1 in MFC–reinforced JP with an increased intensity indicating formation of hydrogen bonding. Since, there was no new peak observed in MFC–reinforced JP resin, it may be concluded that the reinforcement by MFC was not caused by chemical interaction. Rather, it can be attributed to hydrogen bonds or electrostatic interaction between MFC and JP. From the ATR-FTIR analysis, a schematic illustration of MFC–reinforced JP resin has been proposed in Fig. 4 with probable cross-linking and hydrogen bonding among MFC, glyoxal and JP resin.
O stretching increased due to the presence of MFC.39 Also, the peak at 1080 cm−1 observed in the cross-linked JP for C–OH group shifted significantly to 1055 cm−1 in MFC–reinforced JP with an increased intensity indicating formation of hydrogen bonding. Since, there was no new peak observed in MFC–reinforced JP resin, it may be concluded that the reinforcement by MFC was not caused by chemical interaction. Rather, it can be attributed to hydrogen bonds or electrostatic interaction between MFC and JP. From the ATR-FTIR analysis, a schematic illustration of MFC–reinforced JP resin has been proposed in Fig. 4 with probable cross-linking and hydrogen bonding among MFC, glyoxal and JP resin.
Fig. 1 also shows photographic images of pure, plasticized, cross-linked and MFC–reinforced JP resin sheets in water solubility test after 24 h. Because of the increased hydrophilicity for sorbitol, plasticized JP resin sheets showed initial softening along with increased surface area followed by considerable warping and roll forming. However, cross-linked sheets remained nearly intact while MFC–reinforced sheets did not change at all after 24 h water immersion. Cross-linking makes the structure rigid and less expandable and also does not allow it to warp due to the formation of an isotropic 3-dimensional structure. Addition of MFC-reinforcement improved the specimen rigidity even further and restricted warping or roll forming. Even, high temperature (80 °C) agitation for 24 h did not change the physical states of pure and modified JP resin sheets in water. Fig. 2 shows the photographs of the pure and modified JP resin sheets in water after high temperature shaking at 200 rpm for 24 h which reveals a significantly better water stability of JP resin compared to soy protein resin.27
Water absorption (WA) is often a critical issue for protein-based resins due to their hydrophilic nature. For example, soy protein-based resin absorbed about 79% water when immersed in DI water at RT for just 2 h.40 WA behavior of pure and modified JP resin sheets as a function of time is shown in Fig. 5. It is clearly seen that at shorter time periods (t < 14 h), water absorption rate of all specimens is high while a quasi-constant absorption rate or equilibrium absorption is observed at longer times (t > 14 h). About 58% and 67% of water was absorbed within 20 h by pure and plasticized resin, respectively. Cross-linked JP resin had WA of about 53% which is over 21% reduction as compared to plasticized JP resin. MFC–reinforced JP resin showed further reduction in WA, of about 27% (from 67% to 49%), after 20 h, as compared to the plasticized JP resin indicating much lower affinity to water. As explained earlier, lower water affinity of MFC–reinforced JP resin can be attributed to its compact structure as well as no water absorption by MFC itself, both of which retard the water diffusion. As can be expected, MFC–reinforced JP resin showed higher swelling resistance and water stability property which is an added advantage for composite applications.
The TGA thermograms of pure and modified JP resins as shown in Fig. 6(b) have three distinct stages. While the weight loss below 150 °C can be attributed to the evaporation of absorbed water, plasticizer evaporation and thermal degradation of JP resin into small molecules occurred between 200 and 300 °C. The complete carbonization of JP resin occurred from 450 to 600 °C with approximately 20–30% char yield remaining. Among all specimens, plasticized JP resin showed the lowest initial degradation temperature (Td) of 229 °C (Table 3). Clearly, plasticization reduces the thermal stability by interspersing around the polymer molecules and breaking intermolecular interactions. Cross-linking, on the other hand, improved the thermal stability of the plasticized JP resin which can be attributed to the formation of strong imine bonds between glyoxal and JP.12 Similar behavior has been observed in resins based on soy and cottonseed proteins as well.12,27 With the addition of MFC, the thermal stability of the cross-linked JP resin increased further as MFC is thermally more stable than plasticized and cross-linked JP resin.36 Slight improvement in thermal degradation was observed on MFC incorporation as the fibrils stay in a separate phase in the sheets and the difference between their degradation temperatures is not significant. However, significant improvement was observed in MFC–reinforced JP when compared to plasticized JP (p-value < 0.05). Overall, the thermal stability of JP resin was found to be comparable with that of commercial soy protein based resins.27,36,43 In addition, a long-term thermal stability test of MFC–reinforced JP resin was performed by isothermal TGA (ESI: Fig. S1†). Only 4–5% weight reduction was observed in the resin after 30 h at 60 °C which indicates that the resin is thermally stable on a long term basis. Considering the initial weight loss of about over 2% due to the evaporation of moisture, the loss due to isothermal aging is only about 2%. In addition, at 30 h thermal aging the weight loss seems to be stabilizing.
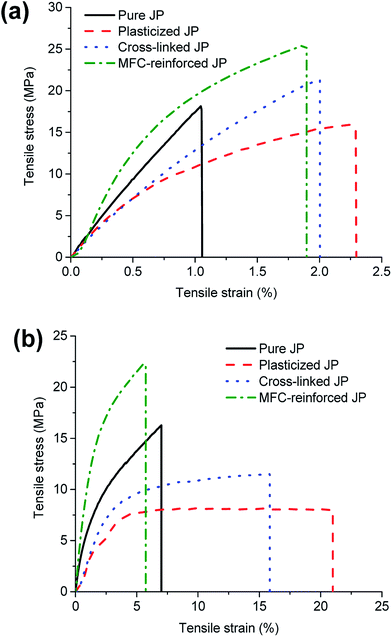 | ||
| Fig. 7 Typical tensile stress–strain responses of pure and modified JP resin in dry (a) and conditioned (b) states. | ||
| Specimen sheets | Young's modulus (MPa) | Tensile strength (MPa) | Fracture strain (%) | Moisture content (%) |
|---|---|---|---|---|
| a Values in parentheses are % coefficient of variation. | ||||
| Pure JP | 720 (11.6)a | 15.1 (16.6)a | 8.2 (32.9)a | 10.8 (6.0)a |
| Plasticized JP | 460 (16.3) | 8.5 (17.6) | 26.3 (21.3) | 12.6 (3.2) |
| Cross-linked JP | 670 (12.3) | 14.5 (14.5) | 15.2 (24.2) | 11.3 (4.5) |
| MFC–reinforced JP | 1147 (11.3) | 20.3 (14.3) | 6.3 (38.0) | 8.7 (4.6) |
In the dry state, the MC values of all resins were observed in the range of 3–4%. Pure JP resin was very brittle with fracture strain of about 1% and did not show any yielding. It showed a high Young's modulus of 2.12 GPa and fracture stress (tensile strength) of 22 MPa. Plasticization had only a small effect (p-value < 0.05) on the fracture strain and tensile strength in the dry state. However, about 34% reductions (from 2.12 GPa to 1.39 GPa) in stiffness was observed after plasticizing the pure JP resin in dry state.
Cross-linking made the plasticized JP network rigid and stronger, as expected. Cross-linking of plasticized JP resin increased its Young's modulus by 11% (from 1.39 GPa to 1.55 GPa) and tensile strength by about 18% (from 16.8 MPa to 19.9 MPa). Significant enhancement was observed for MFC incorporated cross-linked JP resin. MFC–reinforced JP resin showed the highest Young's modulus of 2.27 GPa and strength of 30 MPa, both of which are 47% higher than corresponding values for cross-linked JP resin. This significant increase is the result of the excellent mechanical properties of MFC,44 homogeneous dispersion of MFC, strong web-like rigid network structure of MFC and significant hydrogen bonding between protein and MFC36 resulting in excellent MFC/resin interfacial interaction. Both MFC and protein being hydrophilic and water based, it should be possible to increase the MFC content to higher than the 20% used in this study to obtain even higher tensile properties.
In conditioned state, after exposing to high RH of 65% for 24 h, pure JP resin absorbed over 10% moisture. Plasticization with increased moisture absorption resulted in a significant increase in fracture strain of about 8% and showed yielding behavior that was not seen in pure JP resin in the dry state. Most significant effect of moisture, however, was observed in the case of plasticized JP resin which showed the highest MC value of 12.6%. This high moisture absorption resulted in the lowest Young's modulus of 460 MPa and tensile strength of 8.5 MPa. These values for Young's modulus and strength are lower by about 36% and 43%, respectively, in comparison to pure JP resin. A significant yielding after the initial elastic region and increased fracture strain confirmed the plasticization. Even the cross-linked JP resin showed yielding as a result of moisture absorption. Since there are only two amino acids, arginine and lysine, allow crosslinking, it is likely that the cross-link density is not high enough to prevent yielding. As expected, the MC of cross-linked JP resin was slightly lower at 11.3% compared to plasticized JP resin which had MC of 12.6%. As discussed earlier, tighter and compact network structure due to cross-linking results in higher moisture resistance of the resin. Compact network and lower moisture absorption together increased the Young's modulus and tensile strength of cross-linked JP resin by about 45% and 70% while it decreased fracture strain by about 42%, compared to plasticized JP resin. MFC–reinforced JP resin showed the highest modulus and strength in the conditioned state as well. They also exhibited a quasi-static linear behavior with little necking and reduced fracture strain (∼6%). MC of MFC–reinforced JP resin was the lowest (8.7%) and showed the most significant decrease compared to MC obtained for plasticized JP resin (12.6%). This is expected as MFC, being highly oriented and mostly crystalline, is less hygroscopic than protein.36 In summary, water acted as a very good plasticizer along with sorbitol. The significant synergistic effect of plasticizers was clear from the differences in mechanical properties in the dry state and in the conditioned state (Tables 4 and 5). However, in both cases, MFC–reinforced JP resin showed the best functional performances.
To compare the tensile properties of MFC–reinforced JP with a commercially available edible protein-based resin (e.g. soy protein), MFC–reinforced SPI resin specimens were prepared using similar process mentioned in Section 2.4. In dry state, MFC–reinforced SPI resin displayed an average Young's modulus of 2.30 GPa, tensile strength of 48 MPa, and fracture strain of 3.2% while an average Young's modulus value of 1.13 GPa, fracture stress of 30 MPa, and fracture strain of 8.0% were observed in conditioned state (ESI: Fig. S2†). It is clear that the Young's modulus of SPI resin was quite similar to that of JP resin. However, the significant differences in tensile strength between SPI and JP resin were observed due to the differences in protein and fat contents. Once the protein content in JP could be increased to over 90% (as in SPI) by removing fat, it should be possible for JP resin to have similar or better properties compared to SPI resin. Hence, considering the mechanical and other properties described earlier we can conclude that modified JP resin can replace soy or other commercially available edible protein-based resins.
The material property comparison presented by Ashby was utilized (Fig. 8) to show the relative position of MFC–reinforced JP resin in dry and conditioned states with regards to other polymers.45 As can be seen in Fig. 8, MFC–reinforced JP resin in this study is situated in a region of the property space that can be easily comparable with edible protein and starch-based polymeric resin as mentioned by Verbeek and Bier et al.46 Even some engineering polymers such as low density polyethylene, polyurethane, polystyrene, etc., which are used for low-load bearing applications can be comparable with the modified JP resin as represented by Bayer et al.47 JP resin can be easily manipulated by increasing the plasticizer content to increase fracture strain for packaging industries at the expense of stiffness (Young's modulus) while higher MFC content in JP resin can give higher tensile performance for rigid structure-based applications. Thus, a much broader range of mechanical properties can be designed by simply using external modifiers and varying their contents or loading.
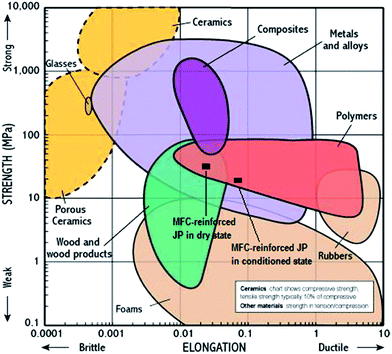 | ||
| Fig. 8 Material property comparison showing strength vs. fracture strain position of MFC–reinforced JP resin represented as black rectangles.45 | ||
4. Conclusions
This study introduces a green approach to developing biodegradable protein-based resin from forestry residue, ‘Jatropha seed cake’ that remains as waste after extracting oil. The use of non-edible Jatropha protein as resin is advantageous over edible proteins due to the fact that Jatropha seed cake is a rich source of protein that has higher molecular weight and reactive amino acids. While the mechanical and moisture resistance properties of Jatropha protein resin are comparable or better than other proteins such as soy protein, as shown in this study, a broad range of tensile properties can be engineered, with the help of additives, which would make Jatropha protein suitable for various packaging and structural applications. Thus, this study opens up new avenues for utilizing waste residues from forestry seed as a sustainable and ‘green’ polymer to replace some petroleum-derived as well as edible biobased polymers in fiber reinforced composites.Acknowledgements
The authors would like to acknowledge NSF-CREST grant (1137681) for funding this research. The authors also would like to acknowledge the use of Cornell Center for Materials Research (CCMR) facilities supported by NSF (award no. DMR-1120296).References
- US EPA, O. Plastics, Common Wastes & Materials, http://www.epa.gov/osw/conserve/materials/plastics.htm, accessed March 10, 2016.
- A. N. Netravali and S. Chabba, Mater. Today, 2003, 6, 22–29 CrossRef.
- D. Lithner, Å. Larsson and G. Dave, Sci. Total Environ., 2011, 409, 3309–3324 CrossRef CAS PubMed.
- J. G. B. Derraik, Mar. Pollut. Bull., 2002, 44, 842–852 CrossRef CAS PubMed.
- M. N. Belgacem and A. Gandini, Monomers, Polymers and Composites from Renewable Resources, Elsevier, Amsterdam, 1st edn, 2008 Search PubMed.
- X. Hu, P. Cebe, A. S. Weiss, F. Omenetto and D. L. Kaplan, Mater. Today, 2012, 15, 208–215 CrossRef CAS.
- M. Singhvi and D. Gokhale, RSC Adv., 2013, 3, 13558–13568 RSC.
- H. Zhang and G. Mittal, Environ. Prog. Sustainable Energy, 2010, 29, 203–220 CrossRef CAS.
- C. Xia, L. Wang, Y. Dong, S. Zhang, S. Q. Shi, L. Cai and J. Li, RSC Adv., 2015, 5, 82765–82771 RSC.
- S. Chabba, G. F. Matthews and A. N. Netravali, Green Chem., 2005, 7, 576–581 RSC.
- W. R. Newson, F. Rasheed, R. Kuktaite, M. S. Hedenqvist, M. Gällstedt, T. S. Plivelic and E. Johansson, RSC Adv., 2015, 5, 32217–32226 RSC.
- H. B. Yue, J. P. Fernandez-Blazquez, P. S. Shuttleworth, Y. D. Cui and G. Ellis, RSC Adv., 2014, 4, 32320–32326 RSC.
- N. H. C. S. Silva, C. Vilela, I. M. Marrucho, C. S. R. Freire, C. P. Neto and A. J. D. Silvestre, J. Mater. Chem. B, 2014, 2, 3715–3740 RSC.
- M. M. Rahman, A. N. Netravali, B. J. Tiimob, V. Apalangya and V. K. Rangari, J. Appl. Polym. Sci., 2016, 133, 43477, DOI:10.1002/app.43477.
- T. Ghosh Dastidar and A. N. Netravali, Carbohydr. Polym., 2012, 90, 1620–1628 CrossRef CAS PubMed.
- M. M. Rahman, A. N. Netravali, B. J. Tiimob and V. K. Rangari, ACS Sustainable Chem. Eng., 2014, 2, 2329–2337 CrossRef CAS.
- M. Y. Koh and T. I. M. Ghazi, Renewable Sustainable Energy Rev., 2011, 15, 2240–2251 CrossRef CAS.
- D. Fairless, Nature, 2007, 449, 652–655 CrossRef PubMed.
- W. M. J. Achten, L. Verchot, Y. J. Franken, E. Mathijs, V. P. Singh, R. Aerts and B. Muys, Biomass Bioenergy, 2008, 32, 1063–1084 CrossRef CAS.
- H. P. Makkar, G. Francis and K. Becker, J. Sci. Food Agric., 2008, 88, 1542–1548 CrossRef CAS.
- A. I. Hamarneh, H. J. Heeres, A. A. Broekhuis and F. Picchioni, Int. J. Adhes. Adhes., 2010, 30, 615–625 CrossRef CAS.
- F. E. M. O'Brien, J. Sci. Instrum., 1948, 25, 73 CrossRef.
- J.-W. Rhim, A. Gennadios, C. L. Weller, C. Cezeirat and M. A. Hanna, Ind. Crops Prod., 1998, 8, 195–203 CrossRef CAS.
- B. Cuq, N. Gontard, J.-L. Cuq and S. Guilbert, J. Food Sci., 1996, 61, 580–584 CrossRef CAS.
- Y. Lu, L. Weng and L. Zhang, Biomacromolecules, 2004, 5, 1046–1051 CrossRef CAS PubMed.
- D. Saetae, T. Kleekayai, V. Jayasena and W. Suntornsuk, Food Sci. Biotechnol., 2011, 20, 29–37 CrossRef CAS.
- T. G. Dastidar and A. N. Netravali, Green Chem., 2013, 15, 3243–3251 RSC.
- M. M. Rahman and A. N. Netravali, ACS Sustainable Chem. Eng., 2014, 2, 2318–2328 CrossRef CAS.
- H. P. S. Makkar and K. Becker, Eur. J. Lipid Sci. Technol., 2009, 111, 773–787 CrossRef CAS.
- G. Wypych, Handbook of Plasticizers, ChemTec Publishing, 2004 Search PubMed.
- T. Mekonnen, P. Mussone, H. Khalil and D. Bressler, J. Mater. Chem. A, 2013, 1, 13379–13398 CAS.
- M. M. Rahman, K. Ho and A. N. Netravali, J. Appl. Polym. Sci., 2015, 132, 41291, DOI:10.1002/app.41291.
- H.-B. Yue, Y.-D. Cui, P. S. Shuttleworth and J. H. Clark, Green Chem., 2012, 14, 2009–2016 RSC.
- C. Marquié, J. Agric. Food Chem., 2001, 49, 4676–4681 CrossRef.
- J. H. Woychik, J. A. Boundy and R. J. Dimler, J. Agric. Food Chem., 1961, 9, 307–310 CrossRef CAS.
- X. Huang and A. Netravali, Compos. Sci. Technol., 2009, 69, 1009–1015 CrossRef CAS.
- M. Henriksson, L. A. Berglund, P. Isaksson, T. Lindström and T. Nishino, Biomacromolecules, 2008, 9, 1579–1585 CrossRef CAS PubMed.
- C. Li, J. Luo, Z. Qin, H. Chen, Q. Gao and J. Li, RSC Adv., 2015, 5, 56518–56525 RSC.
- K. K. Pandey, J. Appl. Polym. Sci., 1999, 71, 1969–1975 CrossRef CAS.
- X. Mo and X. Sun, J. Am. Oil Chem. Soc., 2002, 79, 197–202 CrossRef CAS.
- G. Denavi, D. R. Tapia-Blácido, M. C. Añón, P. J. A. Sobral, A. N. Mauri and F. C. Menegalli, J. Food Eng., 2009, 90, 341–349 CrossRef CAS.
- A. Bigi, G. Cojazzi, S. Panzavolta, N. Roveri and K. Rubini, Biomaterials, 2002, 23, 4827–4832 CrossRef CAS PubMed.
- X. Huang and A. N. Netravali, Biomacromolecules, 2006, 7, 2783–2789 CrossRef CAS PubMed.
- A. J. Svagan, M. A. S. Azizi Samir and L. A. Berglund, Biomacromolecules, 2007, 8, 2556–2563 CrossRef CAS PubMed.
- M. F. Ashby, Acta Metall., 1989, 37, 1273 CrossRef CAS.
- C. J. R. Verbeek and J. M. Bier, in A Handbook of Applied Biopolymer Technology: Synthesis, Degradation and Applications, RSC Green Chemistry (Book 12), Royal Society of Chemistry, 2011, ch. 7 Search PubMed.
- I. S. Bayer, S. Guzman-Puyol, J. A. Heredia-Guerrero, L. Ceseracciu, F. Pignatelli, R. Ruffilli, R. Cingolani and A. Athanassiou, Macromolecules, 2014, 47, 5135–5143 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra07749h |
| This journal is © The Royal Society of Chemistry 2016 |