Fluorescence quenching and spectrophotometric methods for the determination of 6-mercaptopurine based on carbon dots
Yusheng Yuan,
Yalan Wang,
Shaopu Liu,
Yuanfang Li,
Ruilin Duan,
Hui Zhang and
Xiaoli Hu*
Key Laboratory of Luminescent and Real-Time Analytical Chemistry (Southwest University), Ministry of Education, College of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, China. E-mail: xiaolihu@swu.edu.cn
First published on 18th May 2016
Abstract
It is of great significance to develop an eco-friendly and sensitive platform for the detection of 6-mercaptopurine (6-MP) because of its side effects and variable activity. Herein, fluorescence quenching and spectrophotometric methods for the detection of 6-mercaptopurine (6-MP) using carbon dots (CDs) as a fluorescence probe are established. 6-MP not only serves to shelter the CDs effectively from being quenched, but also to reverse the quenching and restore the fluorescence due to its ability to remove Hg2+ from the surface of CDs. After the experimental conditions are optimized, the linear range for the detection of 6-MP is 0.04 to 12 μmol L−1, and the detection limit is 0.01 μmol L−1. When it refers to the spectrophotometric method, the linear range is 0.08 to 12 μmol L−1 and the detection limit is 0.02 μmol L−1. The CDs with 6-MP are systematically characterized with transmission electron microscopy (TEM), ultraviolet-visible (UV-vis) absorption and so on. Furthermore, the proposed methods are not only expected to become a potential tool for the fast response of 6-MP but also possess the potential for practical application to be applied in the determination of 6-MP in human serum samples with satisfactory results.
1. Introduction
6-Mercaptopurine (6-MP), a sulfur analogue of adenine, is one of the main ingredients of anti-neoplastic agents, and uses its toxic properties to suppress cancers such as acute lymphoblastic leukemia and inflammatory bowel diseases.1,2 Different analytical methods have been proposed for the analysis of 6-MP, such as chemiluminescence,3,4 electrochemical methods,5–7 high performance liquid chromatography (HPLC),8–11 mass spectrometry,12 capillary electrophoresis (CE)13 Raman assays,14 and electrochemical assays.5,15 The electrochemical determination of 6-MP has limited application in real samples due to the poor repeatability and complex electrode modification process. HPLC and MS are always used together to detect 6-MP. Although it is selective and sensitive, it always requires expensive equipment and toxic solvents and often involves complex sample pre-treatment. CE and Raman assays also suffer from defects as with HPLC and MS. When it comes to electrochemical assays, these are the most widely reported, and numerous modified electrodes have been used in 6-MP detection. However, their poor repeatability and complexity have limited their application in real samples. Hence it is of great significance to develop a new, convenient, rapid, and sensitive method to detect trace amounts of 6-MP.Carbon dots (CDs), a new class of fascinating carbon material, have attracted considerable attention since their first discovery in 2004.16 In striking contrast to traditional organic dyes and semiconductor quantum dots, CDs are superior fluorescent nanomaterials, and show several prominent advantages, such as low toxicity, excellent biocompatibility, strong and tunable luminescence, and high photostability.17–20 Carbonaceous quantum dots have fuelled intensive research efforts owing to their unique optical properties and their potential applications in sensing,21 imaging,22 and optoelectronic devices.23,24 As alternatives to colloidal semiconductor quantum dots (containing hazardous heavy metals, such as Cd2+), extensive endeavours have thus been made on the development of non- and low-toxic fluorescent nanomaterials, which can be exemplified by the persistent attention to fullerenes,25 carbon nanotubes,26 and graphene oxide.27 However, the limited water solubility, rapid photo-bleaching, and low photoluminescence efficiencies become the main blockage for their broad applications. Among various carbon-based materials, carbon dots (CDs) are particularly encouraging because of their low cost, being easy to get, their photostability, and biocompatibility. Typical fabrication approaches, including laser ablation,28 electrochemical oxidation,29 confined combustion,30 and chemical oxidation,31 are currently regarded as state-of-the-art methods to synthesize CDs, while these methods may involve extreme synthetic conditions, long times and energy consumption, expensive starting materials or apparatus, and inconvenient surface passivation treatments etc. Therefore, an approachable synthesis method suitable for an ordinary laboratory is still urgently in demand. Up to now, CDs used as sensitive fluorescence probes by fluorescence recovery sensing of 6-MP have not been reported yet.
Herein, CDs, as an eco-friendly and sensitive biosensor, were prepared using the hydrothermal method and have been utilized as a fluorescence probe for the detection of 6-MP. The simple detection strategy is presented in Scheme 1. Firstly, the formation of the CDs–Hg2+ ground state complex results in the fluorescence quenching of the CDs, and the fluorescence probe turns off. Then the self-assembly of 6-MP on the CD surfaces occurs, leading to competition between Hg2+ and 6-MP. Furthermore, it disturbs the interaction between Hg2+ and the CDs. When Hg2+ was removed from the surface of the CDs, the fluorescence of the CDs recovers, therefore, the system thus could be turned on. The intensity of the fluorescence recovery and absorbance of the CDs were both in a linear relationship with the concentration of 6-MP, which made it possible to detect 6-MP. The methods showed the advantages of high sensitivity, good selectivity and easy operation. Additionally, the preparation of CDs without the use of toxic raw materials made the probe more eco-friendly, which was pivotal for practical applications. Most importantly, the proposed methods have great potential application in the detection of 6-MP in human serum samples with satisfactory results.
2. Experimental
2.1. Instrumentation
The fluorescence spectrum was recorded with an F-2500 fluorescence spectrophotometer (Hitachi, Tokyo, Japan) using a 1 cm path length. A UV-2450 spectrophotometer (Shimadzu, Japan) was used for acquiring absorption spectra and measuring absorbance. A high resolution transmission electron microscope (Tecnai G2 F20 S-TWIN, FEI Company, USA) was used to characterize the morphology of the CDs, which was operated at an accelerating voltage of 200 kV. Fourier transform infrared spectrometry (FTIR-8400S, Kyoto, Japan) was employed to identify functional groups of the as-prepared CDs. A FL-TCSPC Fluorolog-3 fluorescence spectrometer (Horiba Jobin Yvon Inc., France) is used to measure fluorescence emission decay curves of the systems. A pHS-3D pH meter (Shanghai Scientific Instruments Company, China) was used to measure the pH values.2.2. Reagents
The stock solution of sodium citrate was obtained from Jinshan Chemical Reagent Factory, Chengdu, China, 6-mercaptopurine from Shanghai Aladdin Reagent Co., Ltd, China, and NH4HCO3 from Kelon Chemical Reagent Factory, Chengdu, China. The Britton–Robinson (BR) buffer solutions at different pH values were prepared by mixing the mixed acid (composed of 2.71 mL 85% H3PO4, 2.36 mL HAc and 2.47 g H3BO3) with 0.2 mol L−1 NaOH in different proportions. The buffer solutions were used to control the acidity. All reagents were of analytical reagent grade and were used without further purification, and doubly-distilled water was used throughout.2.3. Synthesis of fluorescent CDs
The pristine CDs were fabricated according to a hydrothermal method.32 Briefly, 0.2 g sodium citrate, 1.5 g NH4HCO3 and 10 mL double-distilled water were put into a Teflon-lined stainless steel autoclave. Furthermore, the mixture was hydrothermally treated for 4 h at 180 °C in a drying oven. After cooling to room temperature, the transparent product was subjected to dialysis (1000 Da, molecular weight cut-off) against ultrapure water for 12 h in order to obtain pure CDs. The purified homogeneous CDs were thus obtained, which were stable for several months. The final product was re-dissolved to 250 mL with double-distilled water and stored at 4 °C for further analysis.2.4. Recommended procedure for determination of 6-mercaptopurine
The procedure for 6-mercaptopurine (6-MP) detection is described as follows: 0.5 mL CDs, 0.5 mL Hg2+ (with a final concentration of Hg2+ of 4 × 10−5 mol L−1) and different amounts of 6-MP were added into a 10 mL colorimetric tube, and diluted to a volume of 5 mL with double-distilled water. Fluorescence spectra were measured at room temperature at λex = 358 nm, and λem = 442 nm. Both the excitation and emission slit width was 10 nm.2.5. Analysis of real samples
The human plasma samples, obtained from different healthy people in a local hospital, were evaluated to verify the accuracy of the proposed method. The analysis of real samples diluted 500 times with ultrapure water was performed. 0.1 mL of the sample was added into a 10.0 mL calibrated tube and diluted to 5.0 mL with ultrapure water. The resultant samples were spiked with standard 6-MP solution at different concentration levels and then analyzed with the proposed method.3. Results and discussion
3.1. Characterization of the CDs
In order to characterize the as-prepared CDs, several methods such as fluorescence spectroscopy, UV-vis absorption spectroscopy, transmission electron microscopy (TEM), and FTIR spectrometry were used to evaluate its characteristics from different aspects. The size and morphology of the as-prepared CDs were observed by TEM. Fig. 1 displays a TEM image of the CDs. It can be clearly seen that the as-synthesized CDs were uniform in size and possess an early spherical shape. The average size of the obtained CDs was about 8 nm, which was comparable to most of the previous work.19,33Fourier transform infrared spectrometry (FTIR) was measured to provide further evidence for the components and the surface functional groups of the as-prepared CDs. As shown in Fig. 2a, the absorption bands around 3402 cm−1 were accounted for by the stretching vibrations of O–H and those at 2929 cm−1 were related to the stretching vibration of C–H. The peak at 1584 cm−1 corresponded to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations, while C–N stretching vibrations at 1412 cm−1 and 949 cm−1 were attributed to the bending vibrations of C–H. Peaks at 1189 and 728 cm−1 were related to the stretching vibration of C–O and N–H, respectively. Meanwhile, in the Raman spectrum shown in Fig. 2b, the peaks of C–O, C–N, and C–C/C
O stretching vibrations, while C–N stretching vibrations at 1412 cm−1 and 949 cm−1 were attributed to the bending vibrations of C–H. Peaks at 1189 and 728 cm−1 were related to the stretching vibration of C–O and N–H, respectively. Meanwhile, in the Raman spectrum shown in Fig. 2b, the peaks of C–O, C–N, and C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are obvious, which were attributed to the D band (sp3-hybridized) and G band (sp2-hybridized) of the CDs. The D band was associated with the vibrations of carbon atoms with dangling bonds in the termination plane of disordered CDs. The G band corresponded to the E2g mode of the CDs and was related to the vibration of the sp2-hybridized carbon atoms in a two-dimensional hexagonal lattice. The coexistence of both bands indicated that the C dots are amorphous.34,35 Both the FTIR and Raman spectra revealed that multiple functional groups like –COOH and a small amount of N-containing groups were present on the surface of the synthesized CDs, which reveals that the as-prepared CDs were mainly surrounded by carboxylate and hydroxyl groups. Furthermore, a typical X-ray diffraction (XRD) pattern of the CDs presented a diffraction peak centered at 30°, and when comparing peak a with the standard card of carbon, it indicated the formation of carbon nanostructures as amorphous carbon (Fig. 2c).
O are obvious, which were attributed to the D band (sp3-hybridized) and G band (sp2-hybridized) of the CDs. The D band was associated with the vibrations of carbon atoms with dangling bonds in the termination plane of disordered CDs. The G band corresponded to the E2g mode of the CDs and was related to the vibration of the sp2-hybridized carbon atoms in a two-dimensional hexagonal lattice. The coexistence of both bands indicated that the C dots are amorphous.34,35 Both the FTIR and Raman spectra revealed that multiple functional groups like –COOH and a small amount of N-containing groups were present on the surface of the synthesized CDs, which reveals that the as-prepared CDs were mainly surrounded by carboxylate and hydroxyl groups. Furthermore, a typical X-ray diffraction (XRD) pattern of the CDs presented a diffraction peak centered at 30°, and when comparing peak a with the standard card of carbon, it indicated the formation of carbon nanostructures as amorphous carbon (Fig. 2c).
As to the optical properties of CDs, the UV-vis absorption spectrum and fluorescence emission spectrum (Fig. 3) of the CDs were investigated. As shown in Fig. 3, the maximal absorption of the CDs was at 340 nm, which was very different from most of the previous reports.36–38 The fluorescence spectrum of the CDs showed a 442 nm emission peak under excitation of 358 nm, with a Stokes shift of 102 nm. The sodium citrate solution itself was non-emitting when excited at 358 nm, which indicated that the bright blue fluorescence came from the CDs. When excited at 358 nm, the full width at half maximum of the CDs emission spectrum was about 65 nm, which was narrower than those reported (70 nm).39,40 The quantum yield (Φu) of the CDs was calculated using quinine sulfate (Φs = 0.55) as the reference standard.41
 | ||
| Fig. 3 Characteristic optical spectra of CDs. Overlapping of absorption, excitation and emission spectra of CDs in aqueous solution. | ||
3.2. Fluorescence and absorption spectra sensing of 6-MP with CDs as the prober
The fluorescence and absorption spectra of the CDs are shown in Fig. 4, respectively. As shown in the two figures, Hg2+ has a dramatic quenching effect on the fluorescence of CDs. Meanwhile, after 6-MP was added, due to the competition reaction between Hg2+ and 6-MP, the fluorescence intensity and absorption coefficient of the analytical system were gradually enhanced with the increase of the concentration of 6-MP. As a result, trace amounts of 6-MP could be detected based on the enhancement of the fluorescence intensity and absorption coefficient of the analytical system. Herein, the fluorescence of the analytical system after 6-MP was added could not be totally restored. It is because the coordination between Hg2+ and the CDs could not be complete and part of Hg2+ would still inhibit the fluorescence of the CDs. | ||
| Fig. 4 Effects of 6-MP on the fluorescence (A) and absorption (B) spectra of the CDs–Hg2+ system. c(Hg2+): 4 × 10−5 mol L−1; (a)–(f) c(6-MP): 2.0, 4.0, 6.0, 8.0, 10.0, and 12.0 μmol L−1. pH = 7.5. | ||
3.3. Optimum conditions of the reaction
| Foreign substance | Concentration (μg L−1) | Relative error (%) | Foreign substance | Concentration (μg L−1) | Relative error (%) |
|---|---|---|---|---|---|
| NaCl | 292.2 | 1.7 | Glucose | 360.32 | 2.6 |
| KBr | 595.0 | 2.3 | Malt sugar | 720.64 | −2.4 |
| CaCl2 | 554.9 | −2.7 | Sucrose | 684.6 | −3.3 |
| MgCl2 | 476.1 | 3.1 | L-Tyrosine | 906.0 | −2.3 |
| NH4Cl | 267.5 | 1.4 | Phenylalanine | 825.95 | −0.8 |
| CuSO4 | 798.4 | −4.3 | L-Arginine | 871.0 | −4.3 |
| ZnCl2 | 681.5 | 3.5 | Methionine | 746.09 | −4.8 |
| MnSO4 | 755.0 | 3.9 | Glycine | 375.35 | −3.1 |
| NH4Fe (SO4)2 | 964.36 | 4.7 | L-Aspartic acid | 665.5 | −2.2 |
| NaF | 209.95 | 2.4 | Vitamin B1 | 300.81 | 3.3 |
| NiSO4 | 788.52 | −4.6 | Vitamin C | 176.12 | 4.3 |
| CoSO4 | 843.45 | −3.7 | β-Cyclodextrin | 1134.98 | 4.8 |
| CdCl2 | 799.44 | 3.2 | NH4SCN | 380.6 | 3.9 |
| KNO3 | 505.5 | −1.6 | Na2SO3 | 630.2 | 3.6 |
3.4. Mechanism for the recognition of 6-MP
Scheme 1 shows the mechanism of the detection of 6-MP using CDs as fluorescence probes. The surface states of the CDs can be influenced by metal ions, which exhibit a strong effect on the fluorescence intensity of the CDs. Due to this effect, CDs are used to detect Hg2+. Initially, when CDs were free in aqueous solution, they showed a strong fluorescence intensity. However, in the presence of Hg2+, the fluorescence of the CDs was quenched significantly through the charge transfer process, which suggests that Hg2+ binds to CDs and the surface state of the CDs changes. One key feature of 6-MP lies in its thiol group, which makes 6-MP have a strong binding preference toward Hg2+ by forming a Hg2+–S bond. Upon the addition of 6-MP, the competitive interaction between Hg2+, 6-MP, and the CDs disturbs the interaction between Hg2+ and the CDs. Hg2+ was then able to be removed from the surface of the CDs, and thus the fluorescence of the system could be recovered. The different fluorescent responses upon the addition of Hg2+ and 6-MP make the CDs able to be used for 6-MP detection.Herein, in order to clarify the fluorescence quenching mechanism, the interaction of 6-MP with the CDs was studied by spectrofluorometry at two temperatures (288 K and 298 K) (Fig. 7) with the Stern–Volmer equation:42–44
| F0/F = 1 + Kqτ0[Q] = 1 + Ksv[Q] |
 | ||
| Fig. 7 The Stern–Volmer curves of Hg2+-quenched CDs at different temperatures. c(Hg2+): 4 × 10−5 mol L−1. c(6-MP): 2.0, 4.0, 6.0, 8.0, and 10.0 μmol L−1. pH = 7.5. | ||
Fig. 7 shows the Stern–Volmer plots of F0/F versus [Q] at two different temperatures, from which it can be seen that with the rise of temperature, the quenching constant decreases. It can be concluded that the quenching process is probably by static quenching resulting from the formation of the CDs–Hg2+ complex. In order to verify the real quenching process further, the fluorescence lifetime and UV-visible absorption spectra are employed to give some more evidence for the actual quenching process.
A powerful method to distinguish weather it is a dynamic or static quenching mechanism is the fluorescence lifetime. If it is dynamic quenching, τ0/τ = F0/F, and the fluorescence lifetime can be cut down by the quenching medium; if it is static quenching, τ0/τ = 1, and the quenching medium can’t change the fluorescence lifetime of the excitation state fluorescence molecule.45,46 The fluorescence emission decay curves of the CDs and CDs–Hg2+ are performed on a FL-TCSPC Fluorolog-3 fluorescence spectrometer at room temperature, as shown in Fig. 8. The fluorescence lifetimes of these two systems are 1.03 ns and 1.20 ns, respectively, which demonstrates that the quenching of fluorescence of the CDs by Hg2+ is a static quenching process.
 | ||
| Fig. 8 Fluorescence emission decay curves of CDs (a) and the CDs–Hg2+ system (b). c(Hg2+): 4 × 10−5 mol L−1. pH = 7.5. | ||
Meanwhile, the UV-vis absorption spectra of the CDs in the absence and presence of Hg2+ were recorded. As shown in Fig. 9, the absorption spectrum of the CDs (curve a) is quite different from the spectrum of CDs–Hg2+ (curve b), which indicates that Hg2+ attached to the surface of the CDs. Furthermore, when 6-MP was added, the absorption spectrum (curve c) changed, and a bigger valley appeared at 286 nm. This might be because the binding force between the CDs and Hg2+ was weak, while the thiol group of 6-MP makes 6-MP have a strong binding preference toward Hg2+ by forming a Hg2+–S bond. In conclusion, the formation of these binary and ternary complexes could cause absorption spectral changes to different extents.47,48
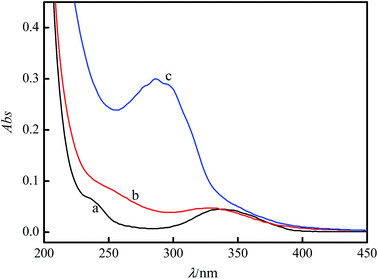 | ||
| Fig. 9 Absorption spectra of the CDs–Hg2+–6-MP system: (a) CDs, (b) CDs–Hg2+, and (c) CDs–Hg2+–6-MP. c(Hg2+): 4 × 10−5 mol L−1. c(6-MP): 8.0 μmol L−1. pH = 7.5. | ||
3.5. Calibration curve
Under the optimum conditions, the linear range for the determination of 6-MP was evaluated by the fluorescence quenching method and spectrophotometry. The intensity of the mixture was enhanced along with elevating the concentrations of the analyte. As shown in Fig. 10A and B, the fluorescence enhancement can also be described by the following equations: ΔF = 688.91c + 43.19 (ΔF = F − F0), and ΔA = 0.333c + 0.043 (ΔA = A − A0). The enhancement of fluorescence is proportional to the concentration of 6-MP in the range from 0.04 to 12 μmol L−1 (R2 = 0.999), with a detection limit of 0.01 μmol L−1 (3σ/k) for the system, while for the spectrophotometry, it was 0.08 to 12 μmol L−1 (R2 = 0.998) and the detection limit was 0.02 μmol L−1 (3σ/k). Obviously, the fluorescence quenching method is much more sensitive than spectrophotometry, but both of them are faster and more facile than the previous works, which are shown in Table 2. | ||
| Fig. 10 Calibration curves for 6-MP: (A) fluorescence, and (B) absorption. c(Hg2+): 4 × 10−5 mol L−1. c(6-MP): 2.0, 4.0, 6.0, 8.0, 10.0, and 12.0 μmol L−1. pH = 7.5. | ||
| Method | Linearity (μmol L−1) | Detection limit (nmol L−1) | Remarks |
|---|---|---|---|
| a FL: fluorescence; EC: electrode chemistry; HPLC: high performance liquid chromatography. | |||
| FLa49,50 | 0.2–66.6 | 75.5 | Rapid, effective, and selective, but the sensitivity is lower |
| 0.08–49.13 | 0.02 | ||
| ECa51–53 | 0.02–0.25 | 0.03 | Low cost and sensitive yet requires much time and several complicated steps to fabricate functionalized electrodes |
| 0.4–100.0 | 0.2 | ||
| 0.5–900.0 | 0.1 | ||
| HPLCa54 | 0.001–0.02 | 0.4 | Effective and selective, but needs expensive equipment, and uses toxic organic solvents |
| Gold nanoparticles55 | 0.1–120.0 | 0.02 | Effective, and selective but the reagents are expensive |
| CdTe quantum dots56 | 0.2–3.2 | 0.08 | Complicated steps and the reagents are toxic |
| FLa (this work) spectrophotometric | 0.04–12.0 | 0.01 | Eco-friendly, fast, facile and sensitive |
| 0.08–3.2 | 0.02 | ||
3.6. Determination of 6-MP in blood serum samples
In order to evaluate the practicality of the proposed methods, blood serum samples were chosen for investigation. The analysis of real samples with 500 times diluted serum samples was performed using the proposed methods. 500 μL sample solution was used based on the procedure described in Section 2.3. The recovery was detected by the standard addition method and the results of the above determination are listed in Table 3, from which it can be seen that the proposed methods had good accuracy (recovery of 98.6–103.0% and 96.7–101.1%, respectively) and repeatability (RSD of 2.8–3.3% and 2.7–3.1%, respectively), and could be successfully applied to the analysis of 6-MP in blood serum samples.| Method | Serum sample | Found (μmol L−1) | Added (μmol L−1) | Total found (μmol L−1) | Recovery (%) | RSD (%, n = 5) |
|---|---|---|---|---|---|---|
| a ND: not found. FL: fluorescence. | ||||||
| Spectrophotometric | 1 | NDa | 0.4 | 0.41 | 102.5 | 2.8 |
| 2 | NDa | 0.7 | 0.68 | 98.6 | 3.1 | |
| 3 | NDa | 1.0 | 1.03 | 103.0 | 3.3 | |
| FLa | 1 | NDa | 0.3 | 0.29 | 96.7 | 2.7 |
| 2 | NDa | 0.6 | 0.59 | 98.3 | 2.9 | |
| 3 | NDa | 0.9 | 0.91 | 101.1 | 3.1 | |
4. Conclusions
In conclusion, fluorescence quenching and spectrophotometric methods for the detection of 6-mercaptopurine (6-MP) using carbon dots (CDs) as a fluorescence probe is established. With the proposed CD-based fluorescence probe, the prepared sensor exhibits a lower detection limit and wider linear range compared with most other 6-MP sensors. Furthermore, this sensor possesses simplicity of preparation, high sensitivity, good stability and reproducibility. The excellent performance of the proposed CD-based fluorescence probe would provide a promising platform for the potential for practical application.Acknowledgements
The authors gratefully acknowledge financial support for this study by grants from the National Natural Science Foundation of China (Grant no. 21575117) and the Special Fund of Chongqing Key Laboratory (CSTC).References
- S. Sahasranaman, D. Howard and S. Roy, Eur. J. Clin. Pharmacol., 2008, 64, 753–767 CrossRef CAS PubMed.
- O. H. Nielsen, B. Vainer and J. Rask-Madsen, Aliment. Pharmacol. Ther., 2001, 15, 1699–1708 CrossRef CAS PubMed.
- X. C. Shen, L. F. Jiang, H. Liang, X. Lu, L. J. Zhang and X. Y. Liu, Talanta, 2006, 69, 456–462 CrossRef CAS PubMed.
- H. W. Sun, T. Wang, X. Y. Liu and P. Y. Chen, J. Lumin., 2013, 134, 154–159 CrossRef CAS.
- P. N. Zhou, L. Q. He, G. L. Gan and S. Y. Ni, J. Electroanal. Chem., 2012, 665, 63–69 CrossRef CAS.
- S. Shahrokhian, F. Ghorbani-Bidkorbeh, A. Mohammadi and R. Dinarvand, J. Solid State Electrochem., 2012, 16, 1643–1650 CrossRef CAS.
- M. Keyvanfarda, V. Khosravi, H. Karimi-Maleh, K. Alizad and B. Rezaei, J. Mol. Liq., 2013, 177, 182–187 CrossRef.
- X. N. Cao, L. Lin, Y. Y. Zhou, G. Y. Shi, W. Zhang, K. Yamamoto and L. T. Jin, Talanta, 2003, 60, 1063–1070 CrossRef CAS PubMed.
- A. F. Hawwa, J. S. Millership, P. S. Collier and J. C. McElnay, J. Pharm. Biomed. Anal., 2009, 49, 401–409 CrossRef CAS PubMed.
- K. M. Naik and S. T. Nandibewoor, J. Anal. Chem., 2013, 68, 1085–1088 CrossRef CAS.
- R. Zakrzewski, K. Borowczyk, A. Luczak, W. Mlynarski, J. Trelinska and A. Luczak, Bioanalysis, 2013, 5, 869–877 CrossRef CAS PubMed.
- J. M. Rosenfeld, V. Y. Taguchi, B. L. Hillcoat and M. Kawai, Anal. Chem., 1977, 49, 725–727 CrossRef CAS PubMed.
- S. R. Rabel, J. F. Stobaugh and R. Trueworthy, Anal. Biochem., 1995, 224, 315–322 CrossRef CAS PubMed.
- H. F. Yang, Y. L. Liu, Z. M. Liu, Y. J. Yang, H. Jiang, Z. R. Zhang, G. L. Shen and R. Q. Yu, J. Phys. Chem. B, 2005, 109, 2739–2744 CrossRef CAS PubMed.
- S. Shahrokhian, F. Ghorbani-Bidkorbeh, A. Mohammadi and R. Dinarvand, J. Solid State Electrochem., 2012, 16, 1643–1650 CrossRef CAS.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- B. Yin, J. Deng, X. Peng, Q. Long, J. Zhao, Q. Lu, Q. Chen, H. Li, H. Tang, Y. Zhang and S. Yao, Analyst, 2013, 138, 6551–6557 RSC.
- S. Qu, X. Wang, Q. Lu, X. Liu and L. Wang, Angew. Chem., Int. Ed., 2012, 124, 12215–12218 CrossRef PubMed.
- P. J. Ni, H. C. Dai, Z. Li, Y. J. Sun, J. T. Hu, S. Jiang, Y. L. Wang and Z. Li, Talanta, 2015, 144, 258–262 CrossRef CAS PubMed.
- Y. Shi, C. Y. Li, S. P. Liu, Z. F. Liu, J. H. Zhu, J. D. Yang and X. L. Hu, RSC Adv., 2015, 5, 64790–64796 RSC.
- Z. Lin, W. Xue, H. Chen and J. M. Lin, Anal. Chem., 2011, 83, 8245–8251 CrossRef CAS PubMed.
- F. Yan, Y. Zou, M. Wang, X. Mu, N. Yang and L. Chen, Sens. Actuators, B, 2014, 192, 488–495 CrossRef CAS.
- J. M. Luther and J. L. Blackburn, Nat. Photonics, 2013, 7, 675–677 CrossRef CAS.
- X. Guo, C. F. Wang, Z. Y. Yu, L. Chen and S. Chen, Chem. Commun., 2012, 48, 2692–2694 RSC.
- K. Xu, F. Liu, J. Ma and B. Tang, Analyst, 2011, 136, 1199–1203 RSC.
- X. L. He, L. Zhang, H. T. Qi, P. Yu, J. J. Fei and L. Q. Mao, Analyst, 2014, 139, 2114–2117 RSC.
- Q. Xi, J. J. Li, W. F. Du, R. Q. Yu and J. H. Jiang, Analyst, 2016, 141, 96–99 RSC.
- H. Goncalves, P. A. S. Jorge, J. R. A. Fernandes and J. C. G. Esteves da Silva, Sens. Actuators, B, 2010, 145, 702–707 CrossRef CAS.
- H. Ming, Z. Ma, Y. Liu, K. Pan, H. Yu, F. Wang and Z. Kang, Dalton Trans., 2012, 41, 9526–9531 RSC.
- A. Rahy, C. Zhou, J. Zheng, S. Y. Park, M. J. Kim, I. Jang and D. J. Yang, Carbon, 2012, 50, 1298–1302 CrossRef CAS.
- Z. A. Qiao, Y. Wang, Y. Gao, H. Li, T. Dai, Y. Liu and Q. Huo, Chem. Commun., 2010, 46, 8812–8814 RSC.
- Y. M. Guo, Z. Wang, H. W. Shao and X. Y. Jiang, Carbon, 2013, 52, 583–589 CrossRef CAS.
- H. L. Tao, X. F. Liao, Q. Y. Wu, X. L. Xie, F. X. Zhong, Z. S. Yi, M. Qin and Z. L. Wu, Spectrochim. Acta, Part A, 2016, 153, 268–272 CrossRef CAS PubMed.
- F. Wang, Z. Xie, H. Zhang, C. Y. Liu and Y. G. Zhang, Adv. Funct. Mater., 2011, 21, 1027–1031 CrossRef CAS.
- J. H. Zhu, M. M. Li, S. P. Liu, Z. F. Liu, Y. F. Li and X. L. Hu, Sens. Actuators, B, 2015, 219, 261–267 CrossRef CAS.
- F. Wang, Y. H. Chen, C. Y. Liu and D. G. Ma, Chem. Commun., 2011, 47, 3502–3504 RSC.
- Y. H. Wang, L. Bao, Z. H. Liu and D. W. Pang, Anal. Chem., 2011, 83, 8130–8137 CrossRef CAS PubMed.
- J. Zong, X. L. Yang, A. Trinchi, S. Hardin, I. Cole, Y. H. Zhu, C. Z. Li, T. Muster and G. Wei, Biosens. Bioelectron., 2014, 51, 330–335 CrossRef CAS PubMed.
- S. S. Wee, Y. H. Ng and S. M. Ng, Talanta, 2013, 116, 71–76 CrossRef CAS PubMed.
- L. P. Lin, X. X. Wang, S. Q. Lin, L. H. Zhang, C. Q. Lin, Z. M. Li and J. M. Liu, Spectrochim. Acta, Part A, 2012, 95, 555–561 CrossRef CAS PubMed.
- D. A. Tekdas, M. Durmus, H. Yanıka and V. Ahsena, Spectrochim. Acta, Part A, 2012, 93, 313–320 CrossRef CAS PubMed.
- R. L. Duan, C. Y. Li, S. P. Liu, Z. F. Liu, Y. F. Li, J. H. Zhu and X. L. Hu, J. Taiwan Inst. Chem. Eng., 2015, 50, 43–48 CrossRef CAS.
- E. Vaishnavi and R. Renganathan, Spectrochim. Acta, Part A, 2013, 115, 603–609 CrossRef CAS PubMed.
- C. X. Hao, S. P. Liu, W. J. Liang, D. Li, L. L. Wang and Y. Q. He, Microchim. Acta, 2015, 182, 2009–2017 CrossRef CAS.
- Y. Guan, W. Zhou, X. H. Yao, M. P. Zhao and Y. Z. Li, Anal. Chim. Acta, 2006, 570, 21–28 CrossRef CAS.
- Y. Y. Yuan, X. Huang, S. P. Liu, J. D. Yang, R. L. Duan and X. L. Hu, RSC Adv., 2016, 6, 3393–3398 RSC.
- Z. Li, Y. N. Ni and S. Kokot, Biosens. Bioelectron., 2015, 74, 91–97 CrossRef CAS PubMed.
- J. Wang, L. Kong, W. Shen, X. L. Hu, Y. Z. Shen and S. P. Liu, Anal. Methods, 2014, 6, 4343–4352 RSC.
- X. Y. Li, Z. Shang, H. Wei and J. D. Yang, Luminescence, 2013, 28, 294–301 CrossRef CAS PubMed.
- L. Wang and Z. J. Zhang, Talanta, 2008, 76, 768–771 CrossRef CAS PubMed.
- H. W. Sun, T. Wang, X. Y. Liu and P. Y. Chen, Luminescence, 2013, 134, 154–159 CrossRef CAS.
- X. N. Cao, L. Lin, Y. Y. Zhou, G. Y. Shi, W. Zhang, K. Yamamoto and L. T. Jin, Talanta, 2003, 60, 1063–1070 CrossRef CAS PubMed.
- M. Keyvanfarda, V. Khosravi, H. Karimi-Maleh, K. Alizad and B. Rezaei, J. Mol. Liq., 2013, 177, 82–187 Search PubMed.
- A. P. Li, J. D. Peng, M. Q. Zhou and J. Zhang, Spectrochim. Acta, Part A, 2016, 154, 1–7 CrossRef CAS PubMed.
- Z. Li, Y. N. Ni and S. Kokot, Biosens. Bioelectron., 2015, 74, 91–97 CrossRef CAS PubMed.
- M. X. Gao, J. L. Xu, Y. F. Li and C. Z. Huang, Anal. Methods, 2013, 5, 673–677 RSC.
| This journal is © The Royal Society of Chemistry 2016 |






