A novel magnetic calcium silicate/graphene oxide composite material for selective adsorption of acridine orange from aqueous solutions†
Abstract
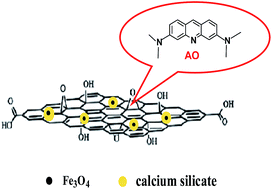
A novel magnetic calcium silicate graphene oxide composite adsorbent (MGSi) was synthesized using a two-phase coprecipitation method. MGSi adsorbent was characterized by transmission electron microscopy (TEM), field emission transmission electron microscopy (HRTEM), X-ray powder diffraction (XRD) and Fourier transform infrared spectrometry (FTIR). The results showed that the coated Fe3O4 nanoparticles with calcium silicate were firmly immobilized on the surface of graphene oxide, and were not easy fall off in the adsorption process compared to normal magnetic graphene oxide (MG) synthesized using the coprecipitation method. The MGSi composite exhibited selective adsorption of the alkaline dye. Acridine orange (AO) was the selected target to be absorbed by MGSi, and batch studies were conducted to evaluate the effects of regulating various parameters, such as the contact time, temperature, initial concentration and solution pH, on the adsorption performance of AO. The adsorption of AO followed pseudo-second-order kinetics, and the equilibrium adsorption was well described by the Freundlich isotherm model. The results of repeated adsorption–desorption cycles indicated that MGSi can be used repeatedly for three cycles. Therefore, the adsorbent could be effectively used to remove AO from wastewater.

 Please wait while we load your content...
Please wait while we load your content...