A graphene oxide incorporated TiO2 photoanode for high efficiency quasi solid state dye sensitized solar cells based on a poly-vinyl alcohol gel electrolyte
Abstract
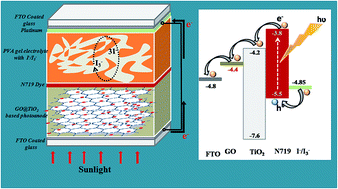
An effective self assembly method was used to synthesize hybrid nanocomposites of TiO2 nanoparticles on graphene oxide (GO) sheets for application in Dye Sensitized Solar Cells (DSSCs). The successful incorporation of TiO2 on the GO sheets was confirmed by X-ray diffraction (XRD), Energy Dispersive X-ray (EDX), Raman and UV-visible spectroscopy. The morphology and size of the TiO2 nanoparticles on the GO sheets were analyzed by scanning electron microscope (SEM) and transmission electron microscope (TEM) analysis. This study is concerned with the effects of different GO contents of the photoanode on the energy conversion efficiency of the PVA gel electrolyte based DSSCs. DSSCs based on the GO@TiO2 nanocomposite photoanode with an optimum GO content of 2.5 wt% showed a short circuit current density (Jsc) of 7.67 mA cm−2, an open circuit voltage (Voc) of 0.76 V and photoconversion efficiency of 3.97% which is much higher than that of the pure TiO2 nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...