A green adsorbent derived from banana peel for highly effective removal of heavy metal ions from water†
Yingchun Li*ab,
Jiang Liua,
Qunhui Yuanc,
Hui Tanga,
Feng Yu*b and
Xin Lv*d
aSchool of Pharmacy, Shihezi University, Shihezi 832000, China. E-mail: yingchunli@shzu.edu.cn; Fax: +86-993-2057005; Tel: +86-993-2057005
bKey Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan, School of Chemistry and Chemical Engineering, Shihezi University, Shihezi 832003, China. E-mail: yufeng05@mail.ipc.ac.cn
cSchool of Materials Science and Engineering, Harbin Institute of Technology (Shenzhen), Shenzhen 518055, China
dKey Laboratory of Oasis Eco-agriculture, Xinjiang Production and Construction Corps, School of Agriculture, Shihezi University, Shihezi 832003, China. E-mail: lxshz@126.com
First published on 4th May 2016
Abstract
A novel carbon foam (CF) was prepared via physically activating banana peel and applied for adsorbing various heavy metal ions including Cu2+, Pb2+, Cd2+ and Cr6+ in aqueous solution. The material was characterized by scanning electron microscopy, Fourier transform infrared spectroscopy and Brunauer–Emmett–Teller analysis. The influences of several variables such as pH, contact time, temperature and initial concentration of ions were investigated through batch experiments. The sorption capacities of our proposed CF are remarkably higher than those of similar products (banana peel-based) from other reports. Kinetic and equilibrium studies illustrate that the sorption behavior can be better described by pseudo-second-order kinetic and Langmuir isotherm models. The maximum sorption capacities of Cu2+, Pb2+, Cd2+ and Cr6+ are estimated to be 49.5, 45.6, 30.7 and 25.2 mg g−1 at an equilibrium time of 5 min. Evaluation of the thermodynamic parameters (ΔG° < 0, ΔH° > 0 and ΔS° > 0) show that the ion sorption onto the CF is an endothermic and spontaneous process. It is noteworthy that the efficiency of the produced material for removing metals can be up to 98% for the contact time of 1 h. The removal efficiency values for 10 kinds of metal ions are 1.3 to 98.6 times higher than those using commercial activated carbon as adsorbent. Among them, the removal efficiency for Cu2+, Pb2+, Cd2+ and Cr6+ is 7.5, 8.9, 8.7 and 16.6 times higher than that of commercial activated carbon, respectively. The obtained material displays a good application prospect in practical wastewater treatment due to its desirable performance, facile preparation and abundant raw material source with a low price.
1. Introduction
In modern society, arbitrary discharge of industrial wastewater containing heavy metal ions has resulted in serious problems like ecological disequilibrium and health hazards; thus effective tactics are urgently needed to abate such pollution. In recent years, developing green, efficient and cheap strategies for heavy metal ion treatment has become an important research issue all over the world. Several techniques, including microbial degradation, bioaccumulation, chemical precipitation, reverse osmosis, ion-exchange, membrane separation, coagulation–flocculation, electro-deposition etc.,1,2 have been used to remove heavy metal ions from aqueous environments. However, these methods have several disadvantages, such as incomplete removal of trace-level heavy metals, consumption of large amounts of chemical reagents and energy, and generation of toxic byproducts that require further treatment.1,3 In comparison, adsorption is generally preferred due to its properties of wide suitability, high efficiency, environmental compatibility, and easy handling and regeneration.4–6Reported adsorption agents mainly include synthetic polymers,7–12 natural minerals (zeolite, kaolin, diatomite, etc.)13–15 and their derivatives.16–18 Synthetic polymers are generally associated with the characteristics of high cost and short life, while low adsorption capacity of natural minerals with limited storage is the bottleneck for large-scale metal removal. Moreover, over-exploitation of natural minerals will bring new environmental problems. Green materials obtained from bio-wastes and agricultural byproducts as adsorbents owing to low cost, abundant sources and easy preparation have been attracting much interest in present studies.19–24 Materials like wood, bark, rice husk, straw, leaf and fruit peel have been reported to play a role in removal of heavy metals and exhibit desirable metal-binding performance.25–29
Banana peel, a common biological waste, is an attractive and inexpensive option and has been employed to get rid of dissolved heavy metals from wastewater but poor removal performance impeded its widespread application.30,31 Some researcher reported the use of banana peel as precursor agents for preparing adsorbents via chemical modification with alkaline and acid.32 However, adsorption capacity only gained limited enhancement after these treatments. Accordingly, new physical and/or chemical modification of banana peel is highly required in order to improve the performance of the obtained adsorbents.
In this work, banana peel was processed by simple calcination treatment and a foam-like carbon material was obtained and adopted to remove Cu2+, Pb2+, Cd2+ and Cr6+ in aqueous solutions. Mechanism of adsorption process was evaluated with several kinetic and isotherm models and thermodynamic parameters were received. Finally, comparison between the carbon foam and commercial activated carbon as adsorbent for heavy metal ions removal was carried out and the results suggest that the removal efficiency of our product is much higher than that of the commercial one. The abundant resources and low cost of banana peel, together with the satisfactory performance of the generated materials makes this study highly valuable and promising in heavy metal treatment.
2. Experimental
2.1. Materials and apparatus
Working solutions including Cu2+, Pb2+, Cd2+ and Cr6+ ions were prepared by diluting high concentration standard stock solutions (1000 mg L−1) that were obtained from Adamas Reagent Co. Ltd. (Shanghai, China). The stock solutions of Na+, K+, Ca2+, Mg2+, Mn2+, Zn2+, Ni2+, Fe3+ ions at the concentration of 1000 mg L−1 were prepared by dissolving NaCl, KCl, CaCl2, MgCl2·6H2O, MnSO4·H2O, ZnSO4·7H2O, NiSO4·6H2O and FeCl3·6H2O in water and then diluted to appropriate concentrations. 0.1 M HCl and 0.1 M NaOH were used for pH value adjustment. All solutions were prepared by using deionized water. Granular activated carbon was obtained from Shanpu chemical co. (Shanghai, China), and ground into powder before use. Banana was obtained from local supermarket. All chemicals are of analytical purity or better and used without further treatment.All the measurement of heavy metal ions were carried out with an AA-7003 flame atomic absorption spectrophotometer (FAAS) (East & West analytical instruments Co., Beijing, China), and the operation followed manufacture manual. pH values were adjusted with a pH-meter (Lei-ci PHS-3C, Inesa-instruments Co., Shanghai, China).
Average pore diameter and specific surface area were measured by a Quantochrome NOVA 1000 based on Brunauer–Emmett–Teller (BET) experiment. Morphology of carbon foam was characterized by scanning electron microscopy (SEM, Zeiss Supra 55VP). X-ray diffraction (XRD) was carried out using PW1710 instrument. Fourier transform infrared (FTIR) spectra were recorded by a Nicolet EXUS-470 FTIR apparatus (Shimadzu, Ja32an).
2.2. Preparation of carbon foam
The raw banana peel was naturally air-dried, collected and stored in a glass dryer prior to use. 1.5 g of air-dried peel was added into 50 mL water and then transferred into a 100 mL Teflon autoclave and hydrothermally treated at 120 °C for 5 h. After filtering and washing with water for three times, the as-prepared peel was freeze-dried at −50 °C for 12 h to get banana peel precursor. The precursor was then calcined at 800 °C for 5 h in Ar atmosphere and banana peel derived carbon foam (BPCF) was obtained. Before use, BPCF was ground into powder.2.3. Adsorption experiments
Adsorption experiments were conducted in 10 mL test tube with a stopper. In isotherm experiments, 20 mg of sorbent was added in 8 mL of Cu2+, Pb2+, Cd2+ and Cr6+ ions solution at desired concentrations (1–1000 mg L−1). Adsorption time was set to be 3 h, if not specified. The effect of pH on adsorption was investigated with pH range from 2.0 to 7.0 and the concentration of metal ions was 5 mg L−1. In kinetic experiments, the sorption time was varied between 0 and 30 min with the metal ion concentration of 5 mg L−1. Once the pre-set time interval reached, the solution was filtered and the metal ion concentration in supernatant was assayed via FAAS. All measurements were performed in triplicate.The adsorption capacity (Q) was calculated by the following equation:
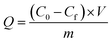 | (1) |
The removal efficiency of metal ions, R%, is calculated from:
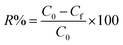 | (2) |
The experimental data from adsorption isotherms were evaluated using Langmuir and Freundlich equations, and pseudo-first and pseudo-second order equations were used to describe the kinetics of the adsorption process. A detailed illustration of the applied mathematical models was given in ESI Tables S1 and S2.†
3. Results and discussion
3.1. Characterization of carbon foam
The prepared BPCF was characterized by using SEM, BET, XRD and FTIR, and the corresponding results were presented in Fig. 1. As shown from the SEM images in Fig. 1A, the material has porous structure on micron scale, and under higher magnification, BPCF displays lamellar morphology like graphene. Typical N2-adsorption–desorption isotherm for BPCF and the corresponding pore size distribution are shown in Fig. 1B. The obtained hysteresis loop in Fig. 1B implies that it is a porous solid adsorption process. BET surface area of BPCF is 456.54 m2 g−1, which is 12.68 times larger than that of untreated banana peel (36 m2 g−1).33 Average pore diameter is 2.77 nm and one can find that BPCF contains micropore (<2 nm), mesopore (2–50 nm) and macropore (50–300 nm). This indicates that the material has relatively large surface area, which is one of the prerequisites as excellent adsorption material.The EDS results show that the essential elements (C, O, P, Cl, K, Mg and Ca) existing in plants were present in the product (Fig. 1C). After adsorbing metal ions, the elements in the adsorbent varied significantly (Fig. 1D). The peaks of heavy metals (Cu, Pb, Cd and Cr) appeared, accompanied with remarkable reduction or disappearance of the original elements of Mg, Ca and K. These phenomena suggest that the main mechanism of adsorption is likely to be an ion exchange process.34
In order to analyze the change of the functional groups in BPCF before and after metal ion sorption and further reveal the sorption mechanism, FTIR was employed and the spectra of BPCF before (curve a) and after adsorbing Cu2+ (curve b) and Pb2+ (curve c), as well as commercial activated carbon (curve d) are displayed in Fig. 2A. The absorption peak at 3455 cm−1 corresponds to the O–H stretching vibration of carboxy and hydroxyl group. The peak observed at 1635 cm−1 may originate from both C–O stretching vibration of carbonyl and carboxy. The peak at 1396 cm−1 can be the flexural vibration of C–H or the vibration of ionic carboxyl groups (–COO−), and the band around 1090 cm−1 might be assigned to the O–H deformation vibration. After metal ion adsorption, one of the main changes in the IR spectra of BPCF was the reduced or disappeared bands around 3455 cm−1, which might be attributed to destruction of O–H stretching vibration of carboxy and hydroxyl groups after adsorbing metals. This suggests that the adsorption of metal ions on BPCF could be based on ion or proton exchange. Compared with activated carbon, BPCF possesses a greater number of hydroxyl and carboxyl groups, implying that BPCF is capable of sorbing larger amount of heavy metals than commercial carbon.
3.2. Effect of pH on metal adsorption
It is known that sorption capacity could be affected by pH value as it is highly related to protonation of sorbent materials.35 The effect of pH on BPCF sorption performance is shown in Fig. 2B. As the initial pH of the solution increased from 2.0 to 7.0, the removal efficiency of Cu2+, Pb2+ and Cd2+ ions presented little change, suggesting that adsorption of these ions on BPCF was basically unaffected by pH values. This can be explained by the fact that the binding sites of BPCF contains not only the pH-susceptible functional groups such as carboxyl and hydroxyl, but also the ion-exchange elements such as “K, Ca, Mg”. The latter is resistant to pH alteration. In addition, we found that addition of BPCF raised pH of solution by 1.5–2.0, which can be ascribed to existence of compounds like CaO, MgO and KOH in BPCF due to calcinations of banana peel. These oxides or hydroxides of alkali metals changed pH when BPCF contacted with water and prompted microprecipitation of heavy metal ion on surface of BPCF. Furthermore, dissolution of these oxides or hydroxides would release heat, and increase of solution temperature will facilitate heavy metal removal by BPCF due to the endothermic nature of the sorption process (the detailed analysis was given in the Section 3.5). The merit that sorbent maintains its adsorption ability in wide pH range is desirable, especially in practical industrial wastewater treatment.It is worth mentioning that, adsorption of Cr6+ was significantly influenced by pH, where the minimum removal efficiency was found to be at pH 2.0 and then increased with pH rising from 2.0 to 4.0. The phenomenon can be explained as follows: (i) there are three hydrate forms of Cr6+ existing in aqueous solution when pH values lower than 6.8, including chromate (CrO42−), dichromate (Cr2O72−) and hydrogen chromate (HCrO4−).36,37 These three ions are all negatively charged, which is unfavorable to bind with deprotonated carboxyl and hydroxyl groups in BPCF owing to electrostatic repulsion. (ii) The size of Cr6+ hydrates is larger than that of free metal ions and therefore they have difficulty in getting through the pores and spreading to the interior of BPCF. (iii) Deprotonation of carboxyl on BPCF was inhibited in the solution with high activity of hydrogen ions, which is detrimental to adsorption of metal cations on BPCF. All the above facts would impair sorption of BPCF toward Cr6+.
As pH value increased, the removal efficiency of adsorbent for chromium ions was elevated, which could be ascribed to decrease in the negative charge density of chromate ions, reduction of ion diameter and ease of deprotonation of carboxyl at higher pH condition.37
3.3. Effect of contact time and kinetic models
The impact of contact time on adsorption of Cu2+, Pb2+, Cd2+ and Cr6+ is shown in Fig. 3A. The equilibrium time for BPCF to remove all metal ions is around 5 min. At adsorption equilibrium, the removal ratio for Cu2+, Pb2+, Cd2+ and Cr6+ at the concentration of 5 mg L−1 is 92.05%, 89.35%, 91.89% and 82.88%, respectively. It is worth mentioning that the removal ratio of BPCF for the four metal ions exceeds 98% at the contact time of 1 h. Compared with other banana-peel adsorbents taking several hours to reach sorption equilibrium,30,38 the BPCF presented in this work exhibits faster adsorption kinetics, which is strongly desirable for high-performance adsorbent. The rapid adsorption could be attributed to large effective surface area and plentiful binding sites and functional groups located in BPCF skeletons. | ||
| Fig. 3 Adsorptions kinetics (A) and adsorption isotherms (B) of Cu2+, Pb2+, Cd2+ and Cr6+ ions by using carbon foam as adsorbent at pH 4.0. | ||
Quantitative kinetic analysis is important for investigating adsorption process and helpful for obtaining an insight of the rate and the limiting step of adsorption, as well as the mechanism. Pseudo-first-order and pseudo-second-order equations were employed to analyze the kinetic adsorption, and the related parameters are listed in Table 1. Based on the values of regression coefficient (R2) of kinetic models, it is found that the pseudo-second-order kinetic model (R2 > 0.997) describes the kinetic sorption data better than the pseudo-first-order kinetic (R2 > 0.794) model does. It is known that pseudo-first-order model is based on the assumption that the adsorption rate is controlled by diffusion of adsorbate while pseudo-second-order model relies on the assumption that the rate-limiting step is chemical sorption involving bonding forces through sharing or exchange of electrons between adsorbate and adsorbent.39 From the experimental results, one can safely grant that the binding behavior of BPCF toward these four ions is a chemical sorption process.
| Metal ions | Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | Qe,cal (mg g−1) | R2 | k2 (g mg−1 min−1) | Qe,cal (mg g−1) | R2 | |
| Cu2+ | 0.350 | 1.245 | 0.902 | 1.855 | 2.075 | 0.999 |
| Pb2+ | 0.349 | 1.287 | 0.794 | 1.808 | 1.181 | 0.997 |
| Cd2+ | 0.381 | 0.937 | 0.808 | 1.873 | 2.458 | 0.999 |
| Cr6+ | 0.284 | 1.142 | 0.886 | 1.724 | 1.016 | 0.999 |
3.4. Effect of initial ion concentration and isotherm models
Isothermal adsorption behavior of BPCF was studied in ion solutions at different initial concentrations ranging from 0 to 1000 mg L−1 at 30 °C, and the experimental adsorption isotherms of Cu2+, Pb2+, Cd2+ and Cr6+ ions are demonstrated in Fig. 3B. It can be seen that the binding capacity (Q) boosts considerably with increase of the concentration of metal ions in initial stage (lower concentration). With continuous rising in metal concentration, increase of Q slows down until finally goes into equilibrium. Langmuir and Freundlich adsorption constants evaluated from the experimental isotherms are listed in Table 2. One can find that Langmuir isotherm gives better fit than the other model, illustrating that adsorption of heavy metal ions on the surface of BPCF are monolayer adsorption and the majority of the binding sites in the sorbent are homogeneously distributed.39–41 According to Langmuir equation, the maximum adsorption capacities (Qmax) for Cu2+, Pb2+, Cd2+ and Cr6+ ions were estimated to be 49.5, 45.6, 30.7 and 25.2 mg g−1, respectively, which are higher than other sorbents derived from banana peel reported in literatures (Table 3).| Metal ions | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qe,cal (mg g−1) | KL (L mg−1) | R2 | n | KF (mg g−1) | R2 | |
| Cu2+ | 49.5 | 0.0048 | 0.997 | 2.315 | 2.246 | 0.941 |
| Pb2+ | 45.6 | 0.0029 | 0.968 | 2.036 | 1.204 | 0.973 |
| Cd2+ | 30.7 | 0.0051 | 0.985 | 2.330 | 1.439 | 0.959 |
| Cr6+ | 25.2 | 0.0037 | 0.986 | 2.014 | 1.782 | 0.861 |
| Adsorbent | Adsorption capacity of metals (mg g−1) | Surface area | Pore diameter | References | |||
|---|---|---|---|---|---|---|---|
| Cu2+ | Pb2+ | Cd2+ | Cr6+ | ||||
| a “—” represents unreported or uninvolved. | |||||||
| Untreated/alkali-hydrolyzed/acid-hydrolyzed/bleached banana peels | — | — | — | 3.36/5.11/4.49/2.46 | — | — | 32 |
| Dried banana peel | — | 7.97 | — | — | — | — | 42 |
| Pulverized banana peel | — | 2.18 | 5.71 | — | — | — | 31 |
| Pulverized banana peel | 28.00 | 7.97 | 5.71 | — | — | — | 43 |
| Minced banana peel | 20.97 | 41.44 | — | — | 2 | 10.5 | 44 |
| Dried/microwave-activated banana peel | — | — | — | 7.22/24.13 | 0.41/1.67 | 2.29/8.03 | 38 |
| Pulverized banana peel | — | 1.70 | 1.69 | 1.66 | — | — | 45 |
| Pulverized banana peel | 52.36 | 25.91 | 34.13 | — | 1.27 | 15.48 | 46 |
| Calcined banana peel | 49.5 | 45.6 | 30.7 | 25.2 | 456.54 | 2.77 | This work |
3.5. Adsorption thermodynamics
Thermodynamic consideration of an adsorption process is necessary to infer whether the process is spontaneous or not, where the Gibbs free energy change (ΔG°) is an important criterion to judge the spontaneity. ΔG° can be calculated from the thermodynamic equilibrium constant, Kc, which is defined as follows:
 | (3) |
 | (4) |
The values of Kc at different temperatures (293–323 K) can be calculated according to the plot of the experimental data of ln(Qe/Ce) versus Qe. Then Gibbs energy change for each temperature can be obtained using the following equation:
ΔG° = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(Kc) ln(Kc)
| (5) |
 | (6) |
Values of Kc, ΔG°, ΔH° and ΔS° are shown in Table 4. The negative values of ΔG° demonstrate that sorption of Cu2+, Pb2+, Cd2+ and Cr6+ ions onto BPCF is spontaneous. The absolute value of ΔG° increased with increasing temperature, indicating that high temperature is beneficial to adsorption of heavy metals. The positive values of ΔH° for all metal ions demonstrate endothermic nature of the sorption process. Also, the positive values of ΔS° imply increased randomness at the solid–liquid interface during adsorption process.
| Metals | Kc | ΔH° (J mol−1) | ΔS° (J mol−1 K−1) | ΔG° (J mol−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | 20 °C | 30 °C | 40 °C | |||
| Cu2+ | 9.1 | 9.85 | 10.7 | 6178 | 39.43 | −5379.36 | −5762.47 | −6168.04 |
| Pb2+ | 1.83 | 3.3 | 5.15 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 399 399 |
139.6 | −1472.11 | −3007.66 | −4265.13 |
| Cd2+ | 2.9 | 6.04 | 11.36 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 029 029 |
186.5 | −2593.64 | −4530.44 | −6323.80 |
| Cr6+ | 1.57 | 1.9 | 2.34 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 227 227 |
55.67 | −1098.82 | −1616.92 | −2212.33 |
3.6. Removal efficiency for general ions and competitive adsorption
To further explore the performance of BPCF, commercial activated carbon was used as a comparison material for metal ions removal with the same experimental operation as BPCF. As shown in Fig. 4A, the removal efficiency of BPCF is 99.95% (Ca2+), 99.84% (Cu2+), 97.53% (Pb2+), 94.69% (Cd2+), 93.72% (Mn2+), 88.02% (Cr6+), 76.06% (Fe3+), 61.14% (Ni2+), 29.60% (Zn2+) and 28.96% (Mg2+). The values are 1.3 to 98.6 times higher than those by using commercial activated carbon as adsorbent. Among them, the removal efficiency for Cu2+, Pb2+, Cd2+ and Cr6+, four deeply concerned metal ions in this research, is 7.5, 8.9, 8.7 and 16.58 times higher than that of commercial activated carbon, respectively. One of the reasons for this result has been discussed in IR characterization; others can be related to the larger specific surface area (456.54 m2 g−1) of BPCF compared with activated carbon (333.88 m2 g−1) and multiple adsorption mechanisms including ion exchange, electrostatic attraction and chemical microprecipitation. Removal efficiency of BPCF varies among different metal ions, which may be due to the different affinities between metal ions and the functional groups in the sorbent, as well as the varying conditions for different metal ions to form hydroxides.36,47–49 Furthermore, BPCF was employed to adsorb Na+ and K+ and it demonstrated very weak clearance toward these two ions. In combination with the above-mentioned EDS results (Fig. 1D, decrease in the amount of K after adsorption of metal ions in BPCF), we can further conclude that the adsorption process is based on ion exchange. | ||
| Fig. 4 The comparison of BPCF and commercial activated carbon for metal ion removal (A). The adsorption behavior of BPCF for metal ions under the coexistence of various metal ions (B). | ||
Considering various metal ions co-existing in industrial wastewater, competitive adsorption performance of the prepared BPCF for metal ion mixture was investigated. 200 mg adsorbent was added in 100 mL of solutions containing mixture of 11 kinds of metal ions at the same concentration of 5 mg L−1, and then the solutions were analyzed by FAAS after 1 h adsorption. The removal efficiency of BPCF under the condition of coexistence of various metal ions is shown in Fig. 4B, and the decrement order of removal efficiency is Fe3+ (77.63%) > Cu2+ (74.41%) > Pb2+ (48.31%) > Ca2+ (26.35%) > Cd2+ (22.82%) > Cr6+ (19.02%) > Ni2+ (4.87%) > Mn2+ (4.10%) > Zn2+ (1.21%) > Mg2+ (0.99%). The results of competitive adsorption are different from that of selective adsorption, which could be related to composite factors including binding energy between BPCF and metal ion, ionic diameter and the formation conditions of hydroxide. The adsorbent can be regenerated by immersion in 0.1 M EDTA for 24 h, and the removal efficiency for metal ions of the regenerated adsorbent is about 80% of that of the original material.
4. Conclusions
In this work, a novel carbon foam was prepared by physical activation of banana peel in a facile and green manner. The banana peel carbon foam (BPCF) was applied for removal of various heavy metal ions (Cu2+, Pb2+, Cd2+ and Cr6+) from aqueous solutions. Compared with other banana peel-based adsorbents, our material illustrates significantly increased adsorption capacity for these four metal ions, which may be accredited to the superior ion exchange capacity and the favorable microprecipitation of metal ions on the surface of BPCF. In the pH range from 2.0 to 7.0, the adsorbent maintained satisfactory clearance rate for most of the heavy metals and the adsorption equilibriums can be obtained within 5–10 min, reflecting fast adsorption kinetics and universal practicability of BPCF. Furthermore, the removal efficiency of our product is several to dozen times higher than that of commercial activated carbon. In conclusion, it is safe to expect that the presented BPCF, featuring high clearance rate, natural and abundant availability and low cost, can serve as an attractive adsorbent for removal of heavy metal ions from wastewater.Acknowledgements
The project financially supported by the Major Program for Science and Technology Development of Shihezi University (gxjs2014-zdgg04), National Natural Science Foundation of China (81260487, 81460543), the Scientific Research Foundation for the Returned Overseas Chinese Scholars from Ministry of Human Resources and Social Security of China (RSLX201301), the Pairing Program of Shihezi University with Eminent Scholar in Elite University (SDJDZ201502), and 2015 Key Technical Innovation Project of Xinjiang Uygur Autonomous Region.Notes and references
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- Q. X. Zhai, F. W. Tian, G. Wang, J. X. Zhao, X. M. Liu, K. Cross, H. Zhang, A. Narbad and W. Chen, RSC Adv., 2016, 6, 5990–5998 RSC.
- Y. Zheng and A. Wang, Chem. Eng. J., 2010, 162, 186–193 CrossRef CAS.
- A. P. Lim and A. Z. Aris, Rev. Environ. Sci. Bio/Technol., 2014, 13, 163–181 CrossRef CAS.
- J. Zhu, S. Wei, M. Chen, H. Gu, S. B. Rapole, S. Pallavkar, T. C. Ho, J. Hopper and Z. Guo, Adv. Powder Technol., 2013, 24, 459–467 CrossRef CAS.
- J. He and J. P. Chen, Bioresour. Technol., 2014, 160, 67–78 CrossRef CAS PubMed.
- J. Zhu, S. Wei, H. Gu, S. B. Rapole, Q. Wang, Z. Luo, N. Haldolaarachchige, D. P. Young and Z. Guo, Environ. Sci. Technol., 2012, 46, 977–985 CrossRef CAS PubMed.
- X. Zuo and R. Balasubramanian, Carbohydr. Polym., 2013, 92, 2181–2186 CrossRef CAS PubMed.
- F. Gao, H. Gu, H. Wang, X. Wang, B. Xiang and Z. Guo, RSC Adv., 2015, 5, 60208–60219 RSC.
- J. Zhu, S. Wei, H. Gu, S. B. Rapole, Q. Wang, Z. Luo, N. Haldolaarachchige, D. P. Young and Z. Guo, Environ. Sci. Technol., 2011, 46, 977–985 CrossRef PubMed.
- H. Gu, S. B. Rapole, J. Sharma, Y. Huang, D. Cao, H. A. Colorado, Z. Luo, N. Haldolaarachchige, D. P. Young, B. Walters, S. Wei and Z. Guo, RSC Adv., 2012, 2, 11007 RSC.
- J. Zhu, H. Gu, J. Guo, M. Chen, H. Wei, Z. Luo, H. A. Colorado, N. Yerra, D. Ding, T. C. Ho, N. Haldolaarachchige, J. Hopper, D. P. Young, Z. Guo and S. Wei, J. Mater. Chem. A, 2014, 2, 2256–2265 CAS.
- S. Sen Gupta and K. G. Bhattacharyya, Phys. Chem. Chem. Phys., 2012, 14, 6698–6723 RSC.
- E. Padilla-Ortega, R. Leyva-Ramos and J. Flores-Cano, Chem. Eng. J., 2013, 225, 535–546 CrossRef CAS.
- M. Dinari, G. Mohammadnezhad and R. Soltani, RSC Adv., 2016, 6, 11419–11429 RSC.
- B. I. El-Eswed, R. I. Yousef, M. Alshaaer, I. Hamadneh, S. I. Al-Gharabli and F. Khalili, Int. J. Miner. Process., 2015, 137, 34–42 CrossRef CAS.
- L. Brown, K. Seaton, R. Mohseni and A. Vasiliev, J. Hazard. Mater., 2013, 261, 181–187 CrossRef CAS PubMed.
- T. Sathvika, Manasi, V. Rajesh and N. Rajesh, RSC Adv., 2015, 5, 107031–107044 RSC.
- A. Bhatnagar, M. Sillanpaa and A. Witek-Krowiak, Chem. Eng. J., 2015, 270, 244–271 CrossRef CAS.
- P. D. Pathak, S. A. Mandavgane and B. D. Kulkarni, Rev. Chem. Eng., 2015, 31, 361–381 CAS.
- A. Demirbas, J. Hazard. Mater., 2008, 157, 220–229 CrossRef CAS PubMed.
- S. Khosravihaftkhany, N. Morad, A. Z. Abdullah, T. T. Teng and N. Ismail, RSC Adv., 2015, 5, 106498–106508 RSC.
- Y. Ding, Y. G. Liu, S. B. Liu, Z. W. Li, X. F. Tan, X. X. Huang, G. M. Zeng, Y. Y. Zhou, B. H. Zheng and X. X. Cai, RSC Adv., 2016, 6, 5223–5232 RSC.
- B. Qiu, H. Gu, X. Yan, J. Guo, Y. Wang, D. Sun, Q. Wang, M. Khan, X. Zhang, B. L. Weeks, D. P. Young, Z. Guo and S. Wei, J. Mater. Chem. A, 2014, 2, 17454–17462 CAS.
- I. Anastopoulos and G. Z. Kyzas, J. Mol. Liq., 2014, 200, 381–389 CrossRef CAS.
- T. A. Nguyen, H. H. Ngo, W. S. Guo, J. Zhang, S. Liang, Q. Y. Yue, Q. Li and T. V. Nguyen, Bioresour. Technol., 2013, 148, 574–585 CrossRef CAS PubMed.
- X. X. Huang, Y. G. Liu, S. B. Liu, X. F. Tan, Y. Ding, G. M. Zeng, Y. Y. Zhou, M. M. Zhang, S. F. Wang and B. H. Zheng, RSC Adv., 2016, 6, 94–104 RSC.
- L. Chen, T. Ji, L. W. Mu, Y. J. Shi, L. G. Brisbin, Z. H. Guo, M. A. Khan, D. P. Young and J. H. Zhu, RSC Adv., 2016, 6, 2259–2269 RSC.
- B. Qiu, Y. Wang, D. Sun, Q. Wang, X. Zhang, B. L. Weeks, R. O'Connor, X. Huang, S. Wei and Z. Guo, J. Mater. Chem. A, 2015, 3, 9817–9825 CAS.
- M. Achak, A. Hafidi, N. Ouazzani, S. Sayadi and L. Mandi, J. Hazard. Mater., 2009, 166, 117–125 CrossRef CAS PubMed.
- J. Anwar, U. Shafique, Z. Waheed uz, M. Salman, A. Dar and S. Anwar, Bioresour. Technol., 2010, 101, 1752–1755 CrossRef CAS PubMed.
- A. Ali and K. Saeed, Desalin. Water Treat., 2015, 53, 3586–3591 CrossRef CAS.
- V. Subramanian, C. Luo, A. M. Stephan, K. S. Nahm, S. Thomas and B. Q. Wei, J. Phys. Chem. C, 2007, 111, 7527–7531 CAS.
- L. Carro, J. L. Barriada, R. Herrero and M. E. S. de Vicente, Chem. Eng. J., 2015, 264, 181–187 CrossRef CAS.
- J. Fan, T. Onal Okyay and D. Frigi Rodrigues, J. Hazard. Mater., 2014, 279, 236–243 CrossRef CAS PubMed.
- H. H. Najafabadi, M. Irani, L. R. Rad, A. H. Haratameh and I. Haririan, RSC Adv., 2015, 5, 16532–16539 RSC.
- H. Gu, S. B. Rapole, Y. Huang, D. Cao, Z. Luo, S. Wei and Z. Guo, J. Mater. Chem. A, 2013, 1, 2011–2021 CAS.
- Z. N. Liu, Y. M. Liu, L. Chen and H. Zhang, Desalin. Water Treat., 2014, 52, 7117–7124 CrossRef CAS.
- J. He, Y. Lu and G. Luo, Chem. Eng. J., 2014, 244, 202–208 CrossRef CAS.
- J. Liu, L. Li, H. Tang, F. Zhao, B. C. Ye, Y. Li and J. Yao, J. Sep. Sci., 2015, 38, 3103–3109 CrossRef CAS PubMed.
- J. Liu, L. Zhang, L. Li Han Song, Y. Liu, H. Tang and Y. Li, J. Sep. Sci., 2015, 38, 1172–1178 CrossRef CAS PubMed.
- G. Annadurai, R. S. Juang and D. J. Lee, Water Sci. Technol., 2003, 47, 185–190 CAS.
- K. Arunakumara, B. C. Walpola and M.-H. Yoon, Korean J. Environ. Agric., 2013, 32, 108–116 CrossRef.
- R. S. D. Castro, L. Caetano, G. Ferreira, P. M. Padilha, M. J. Saeki, L. F. Zara, M. A. U. Martines and G. R. Castro, Ind. Eng. Chem. Res., 2011, 50, 3446–3451 CrossRef CAS.
- E. Sabanovic, M. Memic, J. Sulejmanovic and J. Huremovic, Anal. Lett., 2015, 48, 442–452 CrossRef CAS.
- M. Thirumavalavan, Y. L. Lai and J. F. Lee, J. Chem. Eng. Data, 2011, 56, 2249–2255 CrossRef CAS.
- V. K. Gupta, A. Nayak, B. Bhushan and S. Agarwal, Crit. Rev. Environ. Sci. Technol., 2015, 45, 613–668 CrossRef.
- K. Anitha, S. Namsani and J. K. Singh, J. Phys. Chem. A, 2015, 119, 8349–8358 CrossRef CAS PubMed.
- N. M. Mubarak, J. N. Sahu, E. C. Abdullah and N. S. Jayakumar, Sep. Purif. Rev., 2014, 43, 311–338 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Langmuir and Freundlich equations, and pseudo-first and pseudo-second order equations were given in Tables S1 and S2. See DOI: 10.1039/c6ra07460j |
| This journal is © The Royal Society of Chemistry 2016 |


